Abstract
The minichromosome maintenance complex (MCM2-7) is the putative DNA helicase in eukaryotes, and essential for DNA replication. By applying serial extractions to mammalian cells synchronized by release from quiescence, we reveal dynamic changes to the sub-nuclear compartmentalization of MCM2 as cells pass through late G1 and early S phase, identifying a brief window when MCM2 becomes transiently attached to the nuclear-matrix. The data distinguish 3 states that correspond to loose association with chromatin prior to DNA replication, transient highly stable binding to the nuclear-matrix coincident with initiation, and a post-initiation phase when MCM2 remains tightly associated with chromatin but not the nuclear-matrix. The data suggests that functional MCM complex loading takes place at the nuclear-matrix.
Abbreviations
| NM | = | Nuclear matrix |
| MCM | = | Minichromosome maintenance |
Introduction
The nuclear matrix (NM) is a biochemically defined ribonuclear protein framework in higher eukaryotic cells that persists when chromatin, soluble proteins and lipids are removed.Citation1 Chromatin is periodically attached to the NM specifying its characteristic loop organization in interphase nuclei, with functional protein assemblies immobilized at loop bases. Extensive evidence places DNA replication in proximity to the NM within DNA replication factories; aggregates of replication proteins and multiple co-regulated origins.Citation2 (and reviewed in ref.Citation3).
In cycling cells the nucleus is ‘licensed’ for DNA replication in early G1 phase when the hetero-hexameric MCM2-7 complex associates with chromatin.Citation4 This is dependent on the origin recognition complex (ORC1-6), CDT1 and CDC6. Binding and hydrolysis of ATP by MCM2-7 has been shown to be required for CDT1 release and double hexamer formation.Citation5 CDC45 and GINS bind to chromatin and together with MCM2-7 form the CMG complex, an active DNA helicase.Citation6 MCM loading occurs as the cell passes through the G1/S phase transition, functionally defined by the commencement of DNA synthesis. In eukaryotes, MCM activity is regulated by cyclin-dependent kinases (CDKs) and Dbf4-dependent kinase (DDK) (reviewed in ref.Citation7).
Recently, full replication of plasmid DNA was achieved, independent of origin sequences, using a yeast cell-free system over-expressing multiple initiation proteins.Citation8,9 Thus, initiation has been effectively reconstituted, but the system is not fully defined and the mechanism of site selection is not clear. Intrinsic to this is the functional loading of the MCM helicase complex, which is believed to involve ring opening between MCM subunits 2 and 5.Citation10,11 Exactly how this is achieved on chromatin, and rendered functional by accurate interaction with both template and accessory factors, is not fully understood even in yeast.
In higher eukaryotes where spatial constraints play a role in specifying origins, additional considerations come into play, necessitating a description of the relationship between the MCM complex and the NM. A number of studies have begun to look at this in cell lines engaged in continuous culture, with contradictory results. MCM3 and MCM7 have been reported to be resistant to nuclease digestion and therefore characterized as NM bound.Citation12,13 However, other studies show MCM2, MCM3, MCM5 and MCM7 to be solubilized by nuclease digestion and therefore not NM bound.Citation14-18 None of these studies validate the effect of nuclease by demonstrating release of chromatin-associated control proteins, so must be interpreted accordingly. Furthermore, transient interaction may be masked in asynchronous cells by the bulk fraction of MCM protein, making synchronization essential. Here we show fine temporal resolution of the nuclear binding characteristics of MCM2 as cells pass through late G1 phase, in order to describe the types of interaction that occur during expression, assembly, initiation and beyond. Using murine 3T3 cells that can be manipulated to undergo synchronized passage through G1 phase, without the use of chemical inhibitors, we demonstrate 3 distinct binding states of MCM2 in G1 phase following quiescence. These are i. chromatin binding prior to initiation of DNA replication, ii. transient association with the NM approximately 4 hours later, which we postulate reflects functional loading, and iii. stabilized post-initiation presence on chromatin.
Results
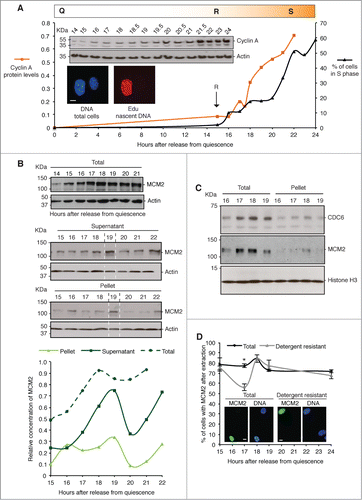
Murine 3T3 cells can be synchronized in quiescence by contact inhibition and serum depletion, then released into cycle as a synchronous wave of cells that pass through G1 landmarks at defined points (). Under these conditions serum-independent S phase entry is triggered at 15 hours after release from quiescence (restriction point, R) and does not vary much between experiments (maximum one hour variance based on appearance of cyclins).Citation19 Entry to S phase is first apparent around one hour later, though most cells in the population enter S phase after 20 hours, reaching a maximum of 60–70% after 24 hours. Cyclin A expression increases steadily over the same period and can be used as a surrogate marker of passage through post-restriction point G1.
Figure 1. Entry to S phase and expression of MCM2. (A) Mouse 3T3 cells re-enter the cell cycle from quiescence in a temporally well-defined manner, passing out of quiescence (Q) and through the restriction point (R) after ∼15 hours. They enter S phase (S) as a wave of cells from 16 hours onwards, with the majority of the population first incorporating labeled nucleotides after 20 hours. The percentage of cells engaged in DNA synthesis (black), and relative concentration of cyclin A protein, estimated by densitometry (averaged from 3 biological replicates) and expressed after normalization to actin (orange), is shown. Inserts (top), show example western blots of cyclin A and actin, and (bottom) micrographs showing incorporation of Edu into newly synthesized DNA in replicating cells (red). DNA is blue. Scale bar is 10 μm. (B) Western blots, and derived quantification, of MCM2 and actin in total cell lysates harvested into CSK buffer (upper), and after separation into detergent-soluble supernatant, and detergent-resistant pellet (nuclei, lower). (C) MCM2, CDC6 and histone H3 in whole cell lysates and detergent resistant pellet (nuclei). CDC6 chromatin binding precedes MCM2 binding. (D) The percentage of cells with MCM2 in the nucleus, detected by immunofluorescence (IF). All labeled cells were scored regardless of intensity, without prior extraction (total MCM2), and after extraction with 0.1% triton X-100 (detergent resistant). Error bars show SEM of 3 replicates (n ≥ 100 for each). Representative images of MCM2 (green) and DNA (blue) are inset. Scale bar is 10 μm.
Expression of MCM2 in late G1 phase
MCM complex proteins are first evident after R, and increase through late G1 (), paralleling the expression of cyclin A. When cells are separated into detergent-soluble and insoluble (nuclei) fractions immediately after harvesting (), a notable peak is detected at 19 hours after release from quiescence, and a short period of instability both before and after this time. This suggests a mechanism that functions to deplete unprotected MCM during this time. Because this temporal profile is not apparent in whole cell lysates, this appears not to act on the bulk of the MCM in the cell, having the greatest impact when cells are disrupted artificially. Chromatin-bound CDC6 does not appear to suffer the same depletion (), though does peak at the same time as chromatin-bound MCM2 (at 18 hours after release from quiescence in this independent experiment). From this data we can say that chromatin binding of CDC6 occurs earlier in G1 than NM-binding of MCM2.
Using an immunofluorescence based measure of the proportion of cells with MCM2, the data show that populations are relatively uniform with a similar number of positive cells at 24 hours after release, as at 15 hours (), in all cases exclusively nuclear. Thus, the quantity increase observed by western blot at 19 hours does not reflect expression in a greater number of cells. Looking specifically at the detergent-resistant fraction of MCM2, a significant fall in numbers is seen at 17 hours, consistent with the suggestion that MCM2 may be unstable at this time.
MCM2 is transiently bound to the NM
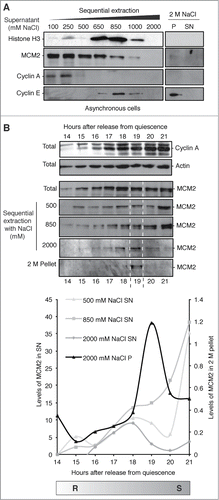
Detergent-resistant proteins are immobilized by interaction with cellular components that are themselves not elutable under physiological buffer conditions; in the nucleus this means chromatin and/or the NM. By increasing NaCl concentration proteins are eluted with different profiles, exemplified here with cyclins A and E,Citation20 and histone H3 (). MCM2 is released gradually from asynchronous cells between 0.1 and 1 M NaCl, describing a heterogeneous population, of which the bulk of MCM2 is unable to resist extraction. However, when applied to synchronized cells, a highly resistant sub-population of MCM2 is evident at 19 hours (), suggesting that MCM2 becomes transiently associated with the NM at this time.
Figure 2. MCM2 is transiently resistant to high-salt extraction. (A) Protein fractions from asynchronous 3T3 cells derived by sequential NaCl washes showing MCM2, cyclins E, and A and histone H3 in the supernatants (SN). 2 M pellet (P) represents the resistant fraction that includes the nuclear-matrix. (B) Protein fractions prepared from synchronized cells in mid G1 to early S phase, using the indicated sequential NaCl concentrations, showing partitioning of MCM2 over time. Total cyclin A and actin are shown for reference. The 19 hour point contains a sub-population of MCM2 that is highly resistant to extraction (NM-associated), indicated by dotted white lines. Graph shows quantification of MCM2 levels from protein gel blots by densitometry (arbitrary units). Levels can be compared within each time course but not between different fractions. Results are shown relative to the lowest time point in all cases, except 2 M pellet fraction which is shown on a different scale to illustrate the peak at 19 hours.
Similar results were obtained using a different NM isolation protocol, in which salt concentration does not exceed 0.5 M, but histones and chromatin-associated proteins are eluted along with DNA fragments after digestion with DNase1. Under these conditions, MCM2 is apparently entirely eluted from asynchronous cells, with none evident in the NM fraction (). However, when this is applied specifically to a 19 hour population a resistant fraction is evident, estimated to be 4% of total MCM2 within the cell at this time (based on densitometry, ). Similar analysis with an extensive set of antibodies raised against other MCM subunits failed to reveal NM-associated populations. This could reflect underlying biology, however we cannot draw a strong conclusion because none of these antibodies are as sensitive as that used to detect MCM2.
Figure 3. MCM2 is transiently resistant to DNase1 extraction. (A) Protein fractions prepared from asynchronous 3T3 cells, and from G1 phase cells harvested at 19 hours, showing MCM2, histone H3 (to reveal efficiency of chromatin digestion) and lamin B2 (to reveal the residual nucleus). Detergent-resistant pellet (P), detergent-soluble supernatant (SN), 0.5 M NaCl wash (W), DNase1-resistant pellet (P, NM indicated with dotted lines), DNase-soluble supernatant (SN, chromatin), mock treatment-resistant pellet (P, NM and chromatin) and mock treatment-soluble supernatant (SN). A fraction of the total MCM2 in the cell resists extraction at 19 hours, but is not detectable in the asynchronous population. (B) Western blots of 0.5 M washed pellets after treatment with DNase1 or mock treatment, from an independent experiment harvested at 14 hours (pre-R), 17 hours (few cells in S phase), 19 hours (most cells initiating) and 20 hours. (C) Percentage of cells with MCM2 in the nucleus after DNase1 (NM) or mock treatment (NM and chromatin), detected by IF (n ≥ 100 for each). Data shown for the 19 hour time point are the average of 2 biological replicates and 3 technical replicates. All other time points show the average of 3 technical replicates. All error bars show SEM. Representative images show MCM2 (green), DNA (blue) and lamin B2 (red). Scale bar is 10 μm. (D) Mean MCM2 fluorescence intensity (left) of DNase1 (n = 100) and mock-extracted (n = 110) 19 hours nuclei, and intensity distribution (right, showing upper bin value).
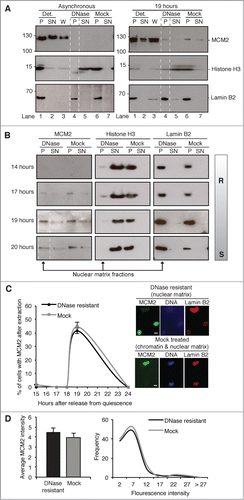
NM isolation by DNase1 extraction was recapitulated over a time course (), focusing on the 0.5 M NaCl-resistant fraction (chromatin and/or NM), generating consistent results, which show a resistant fraction of MCM2 at 19 hours, partially persisting in this time course to 20 hours. At 19 hours, 76% of this immobilized fraction of MCM2 is in fact NM bound (resistant to DNase1 extraction), compared to only 5% of histone H3 and 83% of Lamin B2 (based on densitometry, ). Immunofluorescence analysis of the 0.5 M NaCl-resistant fraction of MCM2 with (NM) and without (NM and chromatin) digestion with DNase1, again identified a resistant fraction at 19 hours (). This shows the number of cells with MCM2 in the nucleus regardless of intensity. The data argue that the 0.5 M resistant fraction that exists at 19 hours reflects the behavior of the majority of cells. No decrease in fluorescence intensity was observed in the chromatin-depleted (NM) population compared to the mock treated (NM and chromatin) population (), showing that all of the protein that resists 0.5 M NaCl is in fact attached to the NM. Together the data argue that even though only a small fraction of MCM2 is resistant to DNase1 (), this is the case for around half of the cells at 19 hours after release from quiescence (). Moreover, as resistance is a transient state, which may in fact last less than an hour, it is likely that more than 50% of the population pass through this state at around this time in late G1 phase.
MCM2 is tightly associated with chromatin after initiation
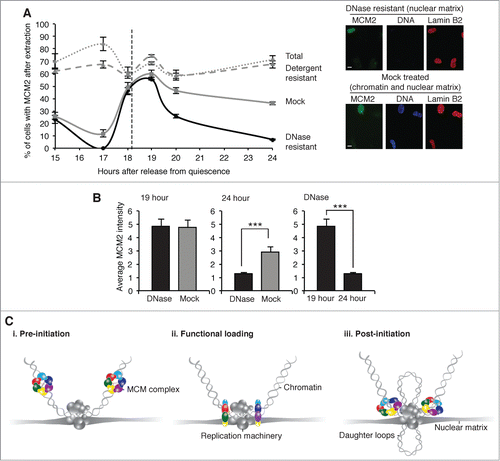
When cells were treated (prior to extraction) with dithiobis succinimidyl propionate (DTSP), a cell-permeable reducing cross-linker which binds proteins to proteins,Citation21 additional binding characteristics were inferred. While the number of nuclei with MCM2, and detergent-resistant MCM2 remained generally high across the time course, the 0.5 M NaCl-resistant fraction reports on distinct time-dependent shifts (). Again a peak is observed at 19 hours, consistent with transient association with the NM, and there was no reduction in fluorescence intensity for MCM2 after depletion of chromatin at this point (). However at 24 hours the response to digestion with DNase1 distinguishes a fraction of MCM2 that is not bound to the NM but is cross-linked to proteins that are themselves tightly-associated with chromatin (possibly stabilization of the heterohexameric ring), and which resists 0.5 M NaCl.
Figure 4. Post-initiation association with chromatin, revealed by crosslinking with DTSP. (A) The percentage of nuclei with MCM2, detected by immunofluorescence, in synchronized, cross-linked populations showing total protein, detergent-resistant protein, DNase1-resistant, and 0.5 M NaCl-resistant protein (mock) (n ≥ 100 for each). Representative images show MCM2 (green), DNA (blue) and lamin B2 (red). Scale bar is 10 μm. (B) Mean MCM2 fluorescence intensity after DNase1 or mock extraction at 19 hours, (n = 99 and 105 respectively, left) and at 24 hours (n = 107 and 101 respectively, center). A comparison of DNase1 resistant MCM2 at 19 and 24 hour is also shown (n = 99 and 107 respectively, right). (C) Schematic showing 3 states of MCM complex binding in late G1 phase, superimposed on the prevailing model of DNA replication in higher eukaryotic nuclei, in which the replication machinery is at chromatin loop bases on the nuclear matrix, with newly synthesized DNA extruded as nascent daughter loops.Citation2 Our data suggest that i. before initiation the MCM complex exists as a chromatin–associated nuclear protein, ii. functional loading immediately prior to initiation takes place coincidently with transient attachment to the NM, iii. after initiation the MCM complex is functionally bound to chromatin but no longer associated with the NM.
Discussion
Abnormalities in DNA replication are linked to disease (reviewed in ref. 22), and in particular to the licensing of DNA for replication (reviewed in ref. 23). Inappropriate licensing can lead to re-replication, replication stress and genomic instability,Citation23 which is a powerful driver toward acquisition of mutations. MCM proteins, as well as other components of the pre-RC, have been shown to be elevated in a range of cancer types,Citation24-33 and MCM complex proteins have been shown to have diagnostic value.Citation34 The mechanistic implications of elevated expression and potential strategies to intervene, hinge on a detailed knowledge of the process as it occurs in mammalian cells. Previous work in yeast,Citation35 Xenopus,Citation4 cancer cells,Citation36,37 and CHO cells,Citation38 all demonstrate stable immobilisation of MCM2 in the nucleus during G1 phase, however these analyses do not further define the binding properties over this crucial period or distinguish NM-bound MCM from chromatin-bound MCM.
Using controlled extraction criteria we have shown a transient relationship between MCM2 and the NM, immediately before the majority of cells first produce nascent DNA. In the cell populations illustrated in approximately 20% have begun to incorporate nascent DNA by 18/19 hours, with the majority delayed by a further 2–3 hours, indicating that NM-association is coincident with or precedes DNA synthesis.
In fact the data show 3 states of MCM binding (); i. resistance to detergent but extraction with DNase1 or 2 M NaCl identifies a chromatin–associated nuclear fraction before 19 hours, ii. resistance to all extraction conditions identifies a transient attachment to the NM at 19 hours, iii. cross-linking to DNase1-sensitive protein that is resistant to 0.5 M NaCl identifies tight association with chromatin after 19 hours. Based on timing in relation to initiation of DNA replication and cyclin A expression (), we suggest that this represents i. pre-initiation chromatin binding, ii. ‘functional loading’, and, iii. post-initiation helicase presence on chromatin. We use the term ‘functional loading’ in order to distinguishing what happens at the NM from chromatin binding. It is clear that MCM2 is bound to chromatin both before and after its transient association with the NM, so NM-association is not likely to reflect loading as defined in most other studies (which do not normally look at the effect of nuclease extraction and so largely report on chromatin binding). It is also clear that chromatin binding can be detected before DNA synthesis, so is not on its own sufficient to support initiation. Our hypothesis is that chromatin binding is converted to a ‘functionally loaded’ state at the NM. However, this may not yet be active helicase as the MCM complex appears to be located away from the NM () and outside of factoriesCitation37 at times when DNA synthesis is detected. Failure of MCM proteins to co-localize with newly synthesized DNACitation39,40 unless analyzed in relation to labeled DNA from the previous cell cycleCitation37 suggests that they are recruited to replication factories prior to initiation, but occupy remote sites during the synthesis phase. Thus, if helicase and polymerase function at the same time they appear not to do it in the same place. It therefore seems unlikely that MCM helicase is ‘activated’ during its brief association with the NM. For this reason we use the term ‘functional loading’ to distinguish this transient and highly extraction-resistant state from the more commonly used descriptions of ‘loading’ and ‘activation’.
The abundance of MCM proteins is far higher than other components of the pre-RC,Citation41 and in excess of the number of activated origins of replication. Although multiple copies appear to be present at each origin, and some are loaded at secondary sites such as those that are activated by replication fork arrest,Citation42,43 only a small fraction of MCM protein appears to be functionally assembled. In yeasts, use of degron mutants confirmed that very small amounts of MCM are required for initiation, but significantly more for elongation.Citation44 A stoichiometric excess of MCM complex may be significant, helping to ensure availability for parallel, synchronous and complete loading at all origins at a specific point in G1 phase. Our data is consistent with this picture, but could also reflect a very brief association with the NM for a far greater proportion of available protein than the 4% detected here (). In fact after the 19 hour loading period MCM2 is in a different state to before 19 hours, and this applies to approximately half of the MCM protein in half of the nuclei quantified. Thus, the data are also consistent with a loading pipeline in which only ∼4% of total MCM2 occupies the loading bay at any one time.
The diffuse nature of replication origins in higher eukaryotic cells (reviewed in ref. 45), argues that structural determinants related to transcription specify their location on the template, while association with an active helicase defines their status as a functional site. The data presented here suggest that functional loading of the MCM complex is specified by activities that are themselves located at the NM, implying that origin selection is governed by template recruitment to these sites. MCM2 has been linked with the NM anchoring protein AKAP95, and disruption of its interaction is shown to inhibit both initiation and elongation of DNA replication, consistent with the idea that NM-association is a requirement for MCM to function in the cellular context.Citation46
In summary, this study adds to the growing evidence that initiation of DNA replication is spatially constrained by immobilization on the NM in mammalian cells, and suggests that functional assembly of the MCM complex occurs during transient presence in NM-associated loading bays. However it does not explain why association is transient, or shed light on the mechanism of loading or the regulation of ring opening. Although the open center of the MCM2-7 hexamer is large enough to accommodate either single stranded DNA or double stranded DNA,Citation47,48 recent studies suggest that when incorporated into the CMG complex it encircles single stranded DNA.Citation49,50 If reconciled with the idea that MCM proteins are located outside of replication factories during DNA synthesis, this implies that template DNA is in single stranded form between the site of DNA synthesis and the site of helicase action. Consistent with our observations, these data also argue that the MCM2-7 hexamer undergoes different conformations and assembly states during the transition from loading to activation.Citation50 Our data identify a specific point in time and location at the NM, offering a direct route to the identification of the factors that spatially constrain the MCM complex and mediate its transition from one state to another at this critical point in the initiation process in mammalian cells.
Materials and methods
Cell culture
Murine 3T3 cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% foetal bovine serum and penicillin/streptomycin/glutamine (10 u/ml, 10 μg/ml, 2.92 mg/ml respectively) on culture dishes (Nunclon), or on glass coverslips, and synchronized in quiescence by contact inhibition and serum depletion as described previously.Citation19 Cells were released into cycle by splitting ¼ into fresh media. Click-iT Edu Cell Proliferation Assay kit (Life Technologies, Cat: C10337) was used as recommended, to analyze the percentage of cells in S phase after a 30 minute labeling period.
Cellular fractionation
Total cell lysates were prepared from adherent cells after rinsing in cold PBS and scrape harvesting into cold cytoskeletal buffer (CSK; 10 mM Pipes pH 6.8, 300 mM sucrose, 100 mM NaCl, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT), with protease inhibitor cocktail (cOmplete®, EDTA-free; Roche) and 2 mM PMSF, as indicated. For separation into detergent-soluble (SN) and detergent-resistant (pellet, P) fractions, lysates were supplemented with 0.1% triton X-100, incubated on ice for 2 minutes and separated by centrifugation. Serial salt extractions were performed as described previously,Citation51 in cold CSK supplemented with protease inhibitors and the indicated concentration of NaCl. Insoluble material was removed by centrifugation after 5 minute incubations on ice in each buffer, and resuspended in a volume of CSK equal to the starting lysate.
NM isolation was carried out as described previously.Citation52 Cell lysates were harvested and supplemented with 0.1% triton X-100, divided into 3 aliquots, and separated into pellet and supernatant by centrifugation. Pellets were washed by resuspension in CSK plus 0.1% triton X-100 and 0.5 M NaCl and salt wash supernatant (W) recovered by centrifugation. Pellets were rinsed in DNase1 buffer (400 mM Tris-HCl, 100 mM NaCl, 60 mM MgCl2, 10 mM CaCl2 pH 7.9), resuspended in the same buffer and incubated at 37°C with DNase1 (RNase free, Roche), or in buffer alone (Mock). After one-hour samples were supplemented with 0.5 M NaCl and separated into SN and P by centrifugation. Cell equivalents were analyzed by protein gel blot.
Western blot analysis
All fractions were immediately boiled in SDS-PAGE sample buffer (240 mM Tris pH 6.8, 8% SDS, 40% glycerol, 0.1% bromophenol blue and 6.8% β mercaptoethanol). Samples were separated by 8% SDS-PAGE, transferred to nitrocellulose or PVDF, blocked with 1 x TBS, 10% dried milk, 0.1% Tween 20, and probed with anti-MCM2 at 1/1000 (BM28, BD Transduction Laboratories), anti-cyclin A at 1/1000 (C4710, Sigma), anti-lamin B2 at 1/1000 (ab138516, Abcam), anti-histone H3 at 1/10 000 (ab1791, Abcam) anti-actin at 1/1000 (AC40, Sigma), anti-cyclin E at 1/500 (ab7959-1, Abcam) or anti-Cdc6 at 1/250 (sc-9964, Santa Cruz Biotech). Secondary antibodies, anti-mouse HRP (ab6789, Abcam) and anti- rabbit HRP (ab6721, Abcam), were used at 1/10 000. Blots were developed using enhanced chemiluminescence (ECL) solution (Amersham). Blots were quantified using NIH ImageJ. Quantification is comparable within each dataset but not between different data sets as all values are relative.
Immunofluorescence
Cells grown on coverslips were washed in PBS, and either fixed immediately in 4% paraformaldehyde (PFA), to visualize ‘total’ protein, or washed with 0.1% triton X-100 in CSK, then PBS before fixing in 4% PFA (detergent resistant samples). DNase1 and Mock samples were washed first in CSK plus 0.1% triton X-100, then CSK plus 0.1% triton X-100 and 0.5 M NaCl, and twice in DNase1 incubation buffer before incubation with DNase1, (RNase free, Roche) diluted in incubation buffer (DNase) or incubation buffer only (Mock), for one hour at 37°C. Cells were further washed in CSK plus 0.1% triton X-100 and 0.5 M NaCl, followed by PBS before fixing in 4% PFA.Citation52 Coverslips were rinsed in PBS, then blocked in 10% BSA, 0.02% SDS, 0.1% triton X-100 in PBS, before incubation with anti-MCM2 at 1/50 (BM28, BD Transduction Laboratories), or anti-Lamin B2 1/100 (ab138516, Abcam). DNA was counterstained with Hoechst 33258. Images were collected using a Zeiss Axiovert 200 M and Openlab image acquisition software, using constant image acquisition parameters. Three technical replicates were analyzed for all samples (n ≥ 99 for each).
Cross-linking
Cells growing on 15 cm dishes were washed 3 times with PBS at room temperature, then incubated in 15 ml cross-link buffer (PBS, 1 mM MgCl2, 0.01% triton X-100) with DTSP (Sigma) at 200 μg/ml, on a rotary shaker for 10 minutes.Citation51 Reactions were quenched with 10 ml 10 mM Tris-HCl pH 7.6, 1 mM EDTA, before extraction as described.
Statistics
Analyses were performed using students T-test, with significance indicated by stars; *** P < 0.001, ** P < 0.01, * P < 0.05. Error bars are standard error of the mean (SEM) in all cases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
ELH designed experiments, acquired and analyzed data and wrote the manuscript. JRPK and RHCW acquired and analyzed data. JPJC advised on design and helped write the manuscript. DC designed experiments, analyzed data and wrote the manuscript.
Acknowledgment
We thank Justin Ainscough for critical comments on the manuscript and Eve Ainscough for graphics.
Funding
This work was funded by Yorkshire Cancer Research (Y002PhD).
References
- Capco DG, Wan KM, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell 1982; 29:847-58; PMID:7151171; http://dx.doi.org/10.1016/0092-8674(82)90446-9
- Cook PR. The nucleoskeleton and the topology of replication. Cell 1991; 66:627-35; PMID:1652367; http://dx.doi.org/10.1016/0092-8674(91)90109-C
- Wilson RH, Coverley D. Relationship between DNA replication and the nuclear matrix. Genes Cells 2013; 18:17-31; PMID:23134523; http://dx.doi.org/10.1111/gtc.12010
- Chong JPJ, Mahbubani HM, Khoo C-Y, Blow JJ. Purification of an MCM-containing complex as a component of the replication licensing system. Nature 1995; 375:418-21; PMID:7760937; http://dx.doi.org/10.1038/375418a0
- Coster G, Frigola J, Beuron F, Morris Edward P, Diffley John FX. Origin licensing requires ATP binding and hydrolysis by the MCM replicative helicase. Mol Cell 2014; 55:666-77; PMID:25087873; http://dx.doi.org/10.1016/j.molcel.2014.06.034
- Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45Mcm2-7GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Nat Acad Sci U S A 2006; 103:10236-41; PMID:16798881; http://dx.doi.org/10.1073/pnas.0602400103
- Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev 2010; 24:1208-19; PMID:20551170; http://dx.doi.org/10.1101/gad.1933010
- Gros J, Devbhandari S, Remus D. Origin plasticity during budding yeast DNA replication in vitro. EMBO J 2014; 33:621-36; PMID:24566988; http://dx.doi.org/10.1002/embj.201387278
- On KF, Beuron F, Frith D, Snijders AP, Morris EP, Diffley JFX. Prereplicative complexes assembled in vitro support origin-dependent and independent DNA replication. EMBO J 2014; 33:605-21; PMID:24566989; http://dx.doi.org/10.1002/embj.201387369
- Lyubimov AY, Costa A, Bleichert F, Botchan MR, Berger JM. ATP-dependent conformational dynamics underlie the functional asymmetry of the replicative helicase from a minimalist eukaryote. Proc Nat Acad Sci U S A 2012; 109:11999-2004; PMID:22778422; http://dx.doi.org/10.1073/pnas.1209406109
- Samel SA, Fernandez-Cid A, Sun J, Riera A, Tognetti S, Herrera MC, Li H, Speck C. A unique DNA entry gate serves for regulated loading of the eukaryotic replicative helicase MCM2-7 onto DNA. Genes Dev 2014; 28:1653-66; PMID:25085418; http://dx.doi.org/10.1101/gad.242404.114
- Fujita M, Ishimi Y, Nakamura H, Kiyono T, Tsurumi T. Nuclear organization of DNA replication initiation proteins in mammalian cells. J Biol Chem 2002; 277:10354-61; PMID:11779870; http://dx.doi.org/10.1074/jbc.M111398200
- Burkhart R, Schulte D, Hu B, Musahl C, Gohring F, Knippers R. Interactions of human nuclear proteins P1Mcm3 and P1Cdc46. Eur J Biochem 1995; 228:431-8; PMID:7705359; http://dx.doi.org/10.1111/j.1432-1033.1995.tb20281.x
- Fujita M, Kiyono T, Hayashi Y, Ishibashi M. In vivo interaction of human MCM heterohexameric complexes with chromatin. Possible involvement of ATP. J Biol Chem 1997; 272:10928-35; PMID:9099751; http://dx.doi.org/10.1074/jbc.272.16.10928
- Stoeber K, Mills AD, Kubota Y, Krude T, Romanowski P, Marheineke K, Laskey RA, Williams GH. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. Embo J 1998; 17:7219-29; PMID:9857179; http://dx.doi.org/10.1093/emboj/17.24.7219
- Cook JG, Chasse DA, Nevins JR. The regulated association of Cdt1 with minichromosome maintenance proteins and Cdc6 in mammalian cells. J Biol Chem 2004; 279:9625-33; PMID:14672932; http://dx.doi.org/10.1074/jbc.M311933200
- Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol 2000; 20:8602-12; PMID:11046155; http://dx.doi.org/10.1128/MCB.20.22.8602-8612.2000
- Cook JG, Park C-H, Burke T, Leone G, DeGregori J, Engel A, Nevins J. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc Natl Acad Sci U S A 2002; 99:1347-52; PMID:11805305; http://dx.doi.org/10.1073/pnas.032677499
- Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol 2002; 4:523-8; PMID:12080347; http://dx.doi.org/10.1038/ncb813
- Munkley J, Copeland NA, Moignard V, Knight JR, Greaves E, Ramsbottom SA, Pownall ME, Southgate J, Ainscough JF, Coverley D. Cyclin E is recruited to the nuclear matrix during differentiation, but is not recruited in cancer cells. Nucleic Acids Res 2011; 39:2671-7; PMID:21109536; http://dx.doi.org/10.1093/nar/gkq1190
- Baumert HG, Fasold H. Cross-linking techniques. Methods Enzymol 1989; 172:584-609; PMID:2546017; http://dx.doi.org/10.1016/S0076-6879(89)72035-8
- Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M. Eukaryotic Chromosome DNA Replication: Where, When and How? In: Kornberg RD, Raetz CRH, Rothman JE, Thorner JW, eds. Annual Review of Biochemistry, Vol 79, 2010:89-130; PMID:20373915; http://dx.doi.org/10.1146/annurev.biochem.052308.103205
- Blow JJ, Gillespie PJ. Replication licensing and cancer - a fatal entanglement? Nat Rev Cancer 2008; 8:799-806; PMID:18756287; http://dx.doi.org/10.1038/nrc2500
- Gonzalez MA, Tachibana KK, Laskey RA, Coleman N. Innovation - Control of DNA replication and its potential clinical exploitation. Nat Rev Cancer 2005; 5:135-41; PMID:15660109; http://dx.doi.org/10.1038/nrc1548
- Hook SS, Lin JJ, Dutta A. Mechanisms to control rereplication and implications for cancer. Curr Opin Cell Biol 2007; 19:663-71; PMID:18053699; http://dx.doi.org/10.1016/j.ceb.2007.10.007
- Williams GH, Stoeber K. Cell cycle markers in clinical oncology. Curr Opin Cell Biol 2007; 19:672-9; PMID:18032010; http://dx.doi.org/10.1016/j.ceb.2007.10.005
- Xouri G, Lygerou Z, Nishitani H, Pachnis V, Nurse P, Taraviras S. Cdt1 and geminin are down-regulated upon cell cycle exit and are over-expressed in cancer-derived cell lines. Eur J Biochem 2004; 271:3368-78; PMID:15291814; http://dx.doi.org/10.1111/j.1432-1033.2004.04271.x
- Lau E, Tsuji T, Guo L, Lu S-H, Jiang W. The role of pre-replicative complex (pre-RC) components in oncogenesis. Faseb Journal 2007; 21:3786-94; PMID:17690155; http://dx.doi.org/10.1096/fj.07-8900rev
- Dudderidge TJ, Kelly JD, Wollenschlaeger A, Okoturo O, Prevost T, Robson W, Leung HY, Williams GH, Stoeber K. Diagnosis of prostate cancer by detection of minichromosome maintenance 5 protein in urine sediments. Brit J Cancer 2010; 103:701-7; PMID:20648010; http://dx.doi.org/10.1038/sj.bjc.6605785
- Lau KM, Chan QKY, Pang JCS, Li KKW, Yeung WW, Chung NYF, Lui PC, Tam YS, Li HM, Zhou L, et al. Minichromosome maintenance proteins 2, 3 and 7 in medulloblastoma: overexpression and involvement in regulation of cell migration and invasion. Oncogene 2010; 29:5475-89; PMID:20661220; http://dx.doi.org/10.1038/onc.2010.287
- Neskoromna-Jedrzejczak A, Tyndorf M, Arkuszewski P, Kobos J. Potential prognostic value of MCM2 expression evaluation in oral cavity squamous cell carcinoma. Wspolczesna Onkologia-Contemp Oncol 2010; 14:196-9
- Kelly JD, Dudderidge TJ, Wollenschlaeger A, Okoturo O, Burling K, Tulloch F, Halsall I, Prevost T, Prevost AT, Vasconcelos JC, et al. Bladder cancer diagnosis and identification of clinically significant disease by combined urinary detection of Mcm5 and nuclear matrix protein 22. Plos One 2012; 7:e40305; PMID:22792272; http://dx.doi.org/10.1371/journal.pone.0040305
- Williams GH, Stoeber K. The cell cycle and cancer. J Pathol 2012; 226:352-64; PMID:21990031; http://dx.doi.org/10.1002/path.3022
- Coleman N, Laskey RA. Minichromosome maintenance proteins in cancer screening. Eur J Cancer (Oxford, England: 1990) 2009; 45 Suppl 1:416-7; PMID:19775653; http://dx.doi.org/10.1016/S0959-8049(09)70071-1
- Donovan S, Harwood J, Drury LS, Diffley JF. Cdc6-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci U S A 1997; 94:5611-6; PMID:9159120; http://dx.doi.org/10.1073/pnas.94.11.5611
- Symeonidou I-E, Kotsantis P, Roukos V, Rapsomaniki M-A, Grecco HE, Bastiaens P, Taraviras S, Lygerou Z. Multi-step loading of human minichromosome maintenance proteins in live human cells. J Biol Chem 2013; 288:35852-67; PMID:24158436; http://dx.doi.org/10.1074/jbc.M113.474825
- Aparicio T, Megias D, Mendez J. Visualization of the MCM DNA helicase at replication factories before the onset of DNA synthesis. Chromosoma 2012; 121:499-507; PMID:22911457; http://dx.doi.org/10.1007/s00412-012-0381-x
- Kuipers MA, Stasevich TJ, Sasaki T, Wilson KA, Hazelwood KL, McNally JG, Davidson MW, Gilbert DM. Highly stable loading of Mcm proteins onto chromatin in living cells requires replication to unload. J Cell Biol 2011; 192:29-41; PMID:21220507; http://dx.doi.org/10.1083/jcb.201007111
- Krude T, Musahl C, Laskey RA, Knippers R. Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication. J Cell Sci 1996; 109:309-18; PMID:8838654
- Dimitrova DS, Todorov IT, Melendy T, Gilbert DM. Mcm2, but not RPA, is a component of the mammalian early G1-phase prereplication complex. J Cell Biol 1999; 146:709-22; PMID:10459007; http://dx.doi.org/10.1083/jcb.146.4.709
- Lei M, Kawasaki Y, Tye BK. Physical interactions among Mcm proteins and effects of Mcm dosage on DNA replication in Saccharomyces cerevisiae. Mol Cell Biol 1996; 16:5081-90; PMID:8756666
- Ge XQ, Jackson DA, Blow JJ. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev 2007; 21:3331-41; PMID:18079179; http://dx.doi.org/10.1101/gad.457807
- Ibarra A, Schwob E, Mendez J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc Nat Acad Sci U S A 2008; 105:8956-61; PMID:18579778; http://dx.doi.org/10.1073/pnas.0803978105
- Liang DT, Hodson JA, Forsburg SL. Reduced dosage of a single fission yeast MCM protein causes genetic instability and S phase delay. J Cell Sci 1999; 112:559-67; PMID:9914167
- Mechali M. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat Rev Mol Cell Biol 2010; 11:728-38; PMID:20861881; http://dx.doi.org/10.1038/nrm2976
- Eide T, Tasken KA, Carlson C, Williams G, Jahnsen T, Tasken K, Collas P. Protein kinase A-anchoring protein AKAP95 interacts with MCM2, a regulator of DNA replication. J Biol Chem 2003; 278:26750-6; PMID:12740381; http://dx.doi.org/10.1074/jbc.M300765200
- Evrin C, Clarke P, Zech J, Lurz R, Sun J, Uhle S, Li H, Stillman B, Speck C. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc Nat Acad Sci U S A 2009; 106:20240-5; PMID:19910535; http://dx.doi.org/10.1073/pnas.0911500106
- Remus D, Beuron F, Tolun G, Griffith JD, Morris EP, Diffley JFX. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell 2009; 139:719-30; PMID:19896182; http://dx.doi.org/10.1016/j.cell.2009.10.015
- Fu YV, Yardimci H, Long DT, The Vinh H, Guainazzi A, Bermudez VP, Hurwitz J, van Oijen A, Schaerer OD, Walter JC. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell 2011; 146:930-40; PMID:21925316; http://dx.doi.org/10.1016/j.cell.2011.07.045
- Costa A, Renault L, Swuec P, Petojevic T, Pesavento JJ, Ilves I, MacLellan-Gibson K, Fleck RA, Botchan MR, Berger JM. DNA binding polarity, dimerization, and ATPase ring remodeling in the CMG helicase of the eukaryotic replisome. Elife 2014; 3:e03273; PMID:25117490; http://dx.doi.org/10.7554/eLife.03273
- Ainscough JF, Rahman FA, Sercombe H, Sedo A, Gerlach B, Coverley D. C-terminal domains deliver the DNA replication factor Ciz1 to the nuclear matrix. J Cell Sci 2007; 120:115-24; PMID:17182902; http://dx.doi.org/10.1242/jcs.03327
- Wilson RHC, Hesketh EL, Coverley D. Preparation of the nuclear matrix for parallel microscopy and biochemical analyses. Cold Spring Harb Protoc 2014; http://dx.doi.org/10.1101/pdb.prot083758
