Abstract
Previous research indicates that the GABAAergic system is involved in the pathophysiology of the fragile X syndrome, a frequent form of inherited intellectual disability and associated with autism spectrum disorder. However, the molecular mechanism underlying GABAAergic deficits has remained largely unknown. Here, we demonstrate reduced mRNA expression of GABAA receptor subunits in the cortex and cerebellum of young Fmr1 knockout mice. In addition, we show that the previously reported underexpression of specific subunits of the GABAA receptor can be corrected in YAC transgenic rescue mice, containing the full-length human FMR1 gene in an Fmr1 knockout background. Moreover, we demonstrate that FMRP directly binds several GABAA receptor mRNAs. Finally, positive allosteric modulation of GABAA receptors with the neurosteroid ganaxolone can modulate specific behaviors in Fmr1 knockout mice, emphasizing the therapeutic potential of the receptor.
Abbreviations
| Act | = | cage activity |
| APRA | = | antibody-positioned RNA amplification |
| ASR | = | acoustic startle response |
| CT | = | C-terminal |
| D/N | = | day/night cycle |
| EMSA | = | electrophoretic mobility shift assay |
| FMRP | = | fragile X mental retardation protein |
| KH | = | hnRNP-K-homology |
| KO | = | knockout |
| MB | = | marble burying |
| mRNP | = | messenger ribonucleoprotein |
| OF | = | open field |
| P22 | = | postnatal day 22 |
| PPI | = | prepulse inhibition |
| QGRS | = | quadruplex forming G-rich sequences |
| RE | = | relative expression |
| RGG | = | arginine-glycine-glycine |
| SoSLIP | = | Sod1 mRNA stem loops interacting with FMRP |
| UTR | = | untranslated region |
| WT | = | wild-type. |
Introduction
Loss-of-function of the FMR1 gene results in the fragile X syndrome, the most common form of inherited intellectual disability and frequently associated with autism spectrum disorder.Citation1 The encoded fragile X mental retardation protein (FMRP) is a selective RNA-binding protein that regulates different aspects of RNA metabolism.Citation1-4 Together with additional proteins and mRNAs, FMRP forms messenger ribonucleoprotein (mRNP) complexes that are transported to specific subcellular locations, including dendrites and dendritic spines, where it associates with the translational machinery to regulate local protein synthesis. In addition, FMRP can control the stability of mRNA targets.Citation5,6
FMRP contains 3 types of RNA-binding domains: 2 hnRNP-K-homology (KH1 and KH2) domains, an arginine-glycine-glycine (RGG) box and the N-terminal region.Citation1,7 The KH2 domain is thought to interact with kissing complex secondary mRNA structuresCitation8 and with short ACUK/WGGA (K=G or U; W=A or U) mRNA sequences.Citation9 The RGG box recognizes G-quadruplexesCitation10,11 and the SoSLIP (Sod1 mRNA stem loops interacting with FMRP) structure.Citation12 Moreover, FMRP binds U-rich mRNA sequences.Citation13 Following the identification of the mRNA-binding properties of FMRP, considerable effort has been made to identify its mRNA targets. Recent large-scale studies suggest that FMRP has several hundreds of putative mRNA targets.Citation9,14 Consequently, absence of FMRP has a profound impact on various neuronal pathways of which the glutamatergic and GABAergic pathway are the most intensively studied.Citation15
In recent years, it has become increasingly clear that both GABAA receptor expression and GABAA receptor-mediated signaling are affected by the absence of FMRP.Citation16-18 We previously reported a reduced expression of several components of the GABAA receptor system in fragile X animal models.Citation19-21 Using real-time PCR, we found a significantly decreased mRNA expression of specific GABAA receptor subunits and key proteins of the GABAergic system in Fmr1 knockout mice. This down-regulation of the GABAA receptor system was also detected in dFmr1 deficient Drosophila melanogaster.
Thus far, the mechanism behind reduced expression of multiple components of the GABAA receptor system remains elusive. The primary objectives of this study were to investigate the molecular link between FMRP and GABAA receptor subunits and to examine the therapeutic potential of the GABAA receptor by means of the positive allosteric modulator ganaxolone.
Results
Reduced GABAA receptor mRNA expression and GABA concentrations in young Fmr1 KO mice
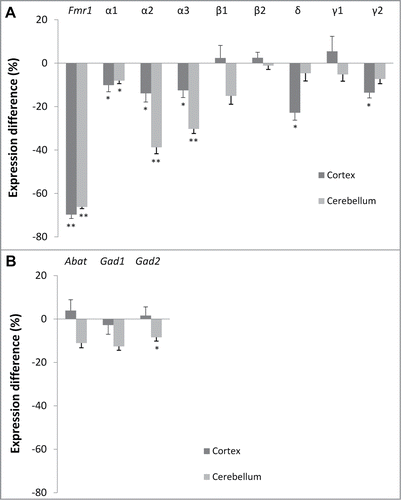
While previous studies have focused primarily on GABAA receptor expression in adult Fmr1 KO miceCitation19,21-23, it has been reported that differential expression varies during development.Citation24 Here, we quantified the mRNA expression levels of specific GABAA receptor subunits (α1, α2, α3, β1, β2, δ, γ1 and γ2), and enzymes involved in GABA metabolism (Abat, Gad1 and Gad2) in the cortex and cerebellum of Fmr1 KO mice and WT littermates at postnatal day 22 (P22). These brain regions were selected because our previous studies in adult mice revealed a reduction in the mRNA expression of GABAA receptor subunits in the cortex of Fmr1 KO mice, but not in the cerebellum.Citation19,21 The mRNA expression levels were measured using quantitative real-time PCR, with normalization using 3 reference genes.Citation25 The reference gene stability was analyzed with qBase software and met the predetermined criteria for stable expression (Table S2). Subsequently, we calculated the relative expression (RE) level, i.e. the ratio of the geometric mean of the normalized expression values of the Fmr1 KO mice to those of WT controls.
The difference in expression between both genotypes is shown in . We observed a significant reduction in α1 (−10%, p = 0.036, Mann-Whitney U test), α2 (−14%, p = 0.021), α3 (−13%, p = 0.036), δ (−23%, p = 0.012) and γ2 (−14%, p = 0.046) in the cortex of Fmr1 KO mice. In contrast to adult mice, significant reductions in mRNA levels were also detected in cerebellar samples of young Fmr1 KO mice. These include reductions in α1 (−8%, p = 0.010), α2 (−39%, p = 0.001) and α3 (−30%, p = 0.001) subunit expression. We did not observe significant differences in the expression of the GABA synthesizing enzymes (Gad1 and Gad2) or the GABA catabolic enzyme (Abat) in the cortex. However, a significant reduction in Gad2 (−8%, p = 0.039) was found in the cerebellum. Finally, Fmr1 mRNA levels were measured as a control and the obtained results (−70%, p = 0.001 in cortex and −66%, p = 0.001 in cerebellum of Fmr1 KO mice) are compatible with previous reports.Citation26
Figure 1. Brain region-specific differences in GABAA receptor subunit and enzyme expression. Difference in normalized expression between Fmr1 KO and WT mice in cortex (n = 8 and n = 8, respectively) and cerebellum (n = 9 and n = 7, respectively) at postnatal day 22. (A) GABAA receptor subunit expression. (B) Enzymes in GABA metabolism. Error bars represent SEM. A Mann-Whitney U test was used to determine the significant differences between both genotypes (*** p < 0.001; **P < 0.01, *P < 0.05).
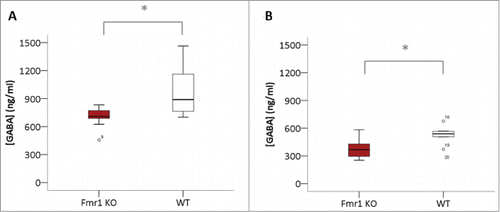
To measure the GABA concentrations in brain homogenates, we performed GABA ELISA experiments in cerebellar and hippocampal samples collected at P22 (). We detected a significant reduction in cerebellar (p = 0.0019, Mann-Whitney U test) and hippocampal GABA concentration (p = 0.035, Mann-Whitney U test).
Figure 2. Reduced GABA concentrations in Fmr1 KO homogenates. GABA concentrations (ng/ml) in homogenates of cerebellum (A) and hippocampus (B) of Fmr1 KO (n=10, gray bars) and WT (n=10, white bars) mice, collected at postnatal day 22. Significant differences were identified with the Mann-Whitney U test (*p < 0.05).
Correction of GABAA receptor deficiency in YAC rescue mice
To establish whether GABAA receptor expression can be restored by reintroduction of FMRP, we analyzed GABAA receptor expression in Fmr1 KO mice in the presence of a transgenic copy of the FMR1 gene. Male YAC transgenic miceCitation27, containing 450 kb of the human Xq27.3 region including the full-length FMR1 gene, were crossed to female Fmr1 heterozygous mice (Fmr1+/−) to generate 4 different genotypes as littermates; WT, WT with the YAC transgene (WT/YAC), Fmr1 KO and Fmr1 KO mice harbouring the YAC transgene (Fmr1 KO/YAC or YAC rescue) (Fig. S1). We used real-time PCR to quantify the mRNA expression of the δ and α1 GABAA receptor subunit in cortical samples of WT, Fmr1 KO and YAC rescue mice at 10 weeks of age. The δ subunit was selected because it was most significantly underexpressed in adult Fmr1 KO mice in our initial studies.Citation19,21 The α1 subunit was also investigated, as it is part of the major GABAA receptor subtype, α1β2γ2, accounting for 43% of all GABAA receptors in the adult brain.Citation28 The reference genes, Gapdh and Hprt, were stably expressed in all 3 investigated genotypes and were used to normalize the mRNA expression levels (Table S4).
We confirmed our initial resultsCitation19 of underexpression of the δ subunit mRNA in Fmr1 KO as compared to WT (67 ± 3% of WT, p = 2.1 × 10−4, Mann-Whitney U test). The reduction in α1 mRNA was borderline significant (87 ± 6% of WT, p = 0.052, Mann-Whitney U test). The expression of both subunits was corrected to WT levels in the YAC rescue mice (δ: 94 ± 3%, p = 0.460, Mann-Whitney U test; α1: 107 ± 3% of WT, p = 0.122, Mann-Whitney U test). represents the relative residual expression of both GABAA receptor subunits in Fmr1 KO and YAC rescue mice as compared to WT littermates (100%).
Figure 3. Correction of α1 and δ mRNA expression in YAC rescue mice. Residual normalized expression of the α1 and δ GABAA receptor subunit mRNAs and Fmr1 mRNA in Fmr1 KO (n=10) and Fmr1 KO/YAC (n = 8; YAC rescue mice) as compared to WT littermates (n = 10). All values are expressed relative to WT levels (100%, white bars), the error bars represent the SEM. A Mann-Whitney U test was used to identify significant differences (#p = 0.052 and ***p < 0.001, significantly different from WT).
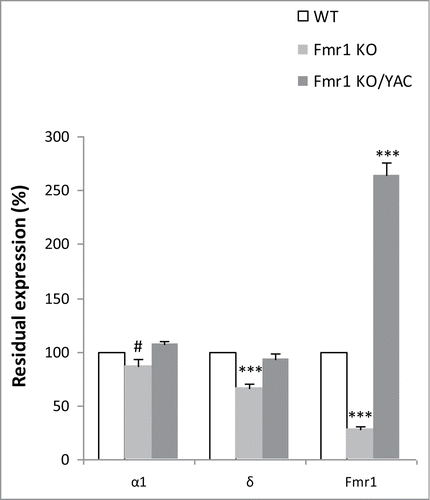
Simultaneously, we verified the Fmr1 expression in the 3 genotypes (). Fmr1 KO mice showed a 28% (28 ± 2% of WT, p = 1.1 × 10−5, Mann-Whitney U test) residual expression of Fmr1 mRNA, the same as previously reported and attributable to the detection of aberrant mRNA.Citation26 FMR1 mRNA is twofold overexpressed in YAC rescue mice (265 ± 11% of WT, p = 4.6 × 10−5, Mann-Whitney U test), in line with previous results.Citation27
FMRP binds directly to GABAA receptor subunit α1 and δ mRNA
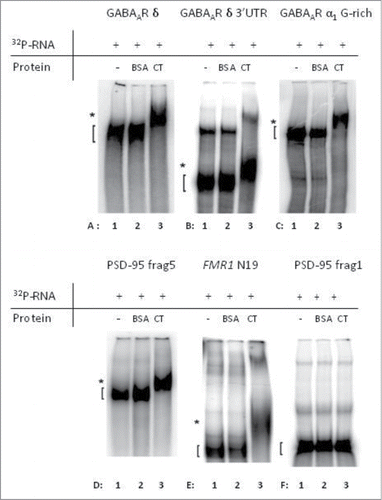
Direct binding between FMRP and specific mRNAs can be shown by EMSA. We focused on the interaction of FMRP with QGRS mRNA sequences.Citation10,11 Putative QGRS were identified in δ and α1 subunit mRNAs using the QGRS Mapper2 program. Based on this in silico analysis (Fig. S2), 6 constructs were selected for the EMSAs (): 3 GABAA receptor subunit mRNAs (entire δ subunit, 3′UTR δ subunit and G-rich part of 3′UTR α1 subunit), 2 positive controls (PSD-95 3′UTR fragment 5, FMR1 – N19) and one negative control (PSD-95 3'UTR fragment 1). The C-terminal part of FMRP contains an RGG box, known to bind to QGRS.Citation10,11 Therefore, EMSAs were performed with the C-terminal part (AA 516-632) of recombinant FMRP and radio-labeled mRNA that was in vitro transcribed from the cDNA constructs ().
Table 1. cDNA constructs used in the EMSAs
Firstly, we performed an EMSA with the entire δ subunit-encoding mRNA (∼1.8 kb) and found evidence for a direct interaction with the C-terminus of FMRP (, compare lanes A1 and A3). Since in silico analysis showed the presence of a G-rich, putative QGRS in this part of the mRNA (Fig. S2), we then focused on the 3′UTR of this mRNA (437 bp). This part of the mRNA showed FMRP-binding ability (). As the entire α1 mRNA is too long (>4kb) to be used in an EMSA, we focused on the G-rich, putative QGRS-region located in the first 601 bp of the 3'UTR (Fig. S2). This region clearly shows a binding to the C-terminus of FMRP (). Positive and negative controls were included to assess the specificity of the assay (). We confirmed binding to known mRNA targets, such as the 3′UTR-fragment 5 of PSD-95 ()Citation5 and fragment N19 of FMR1 ().Citation11 In addition, no binding was observed to the negative control PSD-95 3'UTR-fragment 1 ()Citation5, indicating that the FMRP-RNA interaction was specific and did not simply reflect general RNA affinity.
Figure 4. The C-terminal domain of FMRP is able to specifically interact with mRNA from the indicated subunits of the GABAA receptor. In each electrophoretic mobility shift assay (EMSA), 32P-labeled mRNA-fragments were incubated alone (lane 1), in the presence of BSA (lane 2) or in the presence of the C-terminal (CT) domain of FMRP (lane 3). Direct interaction of the labeled mRNA with FMRP causes a shifted band in the third line (lane A3, B3, C3, D3 and E3) since the protein:RNA complexes migrate more slowly on the polyacrylamide gel. Known targets of FMRP were used as positive controls, PSD-95 3'UTR-fragment 5 (D) Citation5 and fragment N19 of FMR1 (E) Citation11. (F) PSD-95 3'UTR-fragment 1 Citation5 was used a negative control. Unbound RNA-fragments ([) and protein:RNA complexes (*) are indicated.
Ganaxolone modulates behavior of Fmr1 KO mice
A possible treatment strategy for the fragile X syndrome is to enhance GABAergic inhibition by means of positive allosteric modulators of the GABAA receptor.Citation16 Ganaxolone, a synthetic neurosteroid, is such a putative therapeutic compound.Citation29,30 Before exploring the therapeutic effect of ganaxolone, we wanted rule out a possible sedative effect of the drug, which could confound the outcome of behavioral assays. Therefore, the effect of ganaxolone on explorative behavior and overall activity was assessed using the open field and cage activity assay. No significant differences were observed between vehicle-treated Fmr1 KO and WT mice (Figs. S3 and S4). Overall, locomotor activity did not differ between vehicle-treated Fmr1 KO mice and WT mice and was not affected by ganaxolone treatment.
The marble burying assay was used to evaluate the effect of ganaxolone on repetitive and perseverative behavior in Fmr1 KO miceCitation31 and relates to the autistic-like behavior observed in fragile X patients, in particular stereotypic/repetitive behavior.Citation32 Fmr1 KO mice exhibit increased digging behavior, which is reflected by the increased number of marbles that are buried by vehicle-treated Fmr1 KO mice compared to vehicle-treated WT controls (p = 0.001, independent-samples T-test) (). Ganaxolone treatment causes a dose-dependent reduction in the burying behavior of Fmr1 KO mice, without having an effect on WT animals (F(2, 121) = 3.149, p = 0.046, treatment × genotype interaction, 2-way ANOVA). A 5 mg/kg dose had an intermediate effect, not significantly different from vehicle-treated mice (p = 0.331, Tukey post-hoc test), nor from the 10 mg/kg dose (p = 0.465, Tukey post-hoc test). Treatment of Fmr1 KO mice with 10 mg/kg ganaxolone completely corrects the phenotype to the level of WT mice (p = 0.022, Tukey post-hoc test).
Figure 5. Ganaxolone modulates behavior in Fmr1 KO mice. (A) Ganaxolone has a dose-dependent effect on marble burying behavior in Fmr1 KO mice. Vehicle (0 mg/kg body weight) or ganaxolone (5 mg/kg and 10 mg/kg) were administered to Fmr1 KO mice (gray bars) and WT littermates (white bars). The bars indicate the mean number of marbles buried, the error bars represent the SEM. Two-way ANOVA indicates a significant treatment x genotype interaction (F(1,121) = 3.149, p = 0.046). Post-hoc Tukey analysis revealed a significant difference between the 0 mg/kg and 10 mg/kg treatment groups (p = 0.022). Zero dose groups were compared using an Independent samples T-test (p = 0.001). ** p < 0.01, *P < 0.05. n = 18-22/group. (B) The acoustic startle response (N) of Fmr1 KO (gray) and WT (white) mice to a 120 dB stimulus is unaffected by ganaxolone treatment. (C) Three different prepulse intensities were evaluated as shown on the x-axis. Prepulse inhibition (PPI%) is increased in vehicle (VEH)-treated Fmr1 KO compared to vehicle-treated WT mice. Ganaxolone (GAN) treatment (10 mg/kg, i.p.) has an intermediary effect on PPI in Fmr1 KO mice. One-way ANOVA with Tukey post hoc analysis (n: 14-23, * p<0.05).
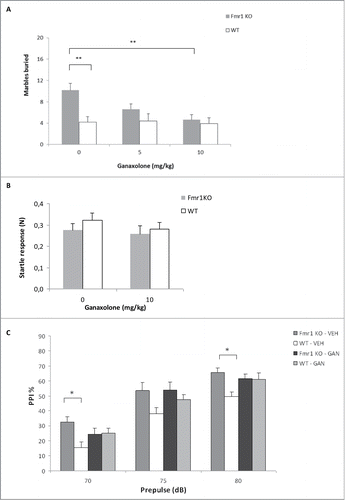
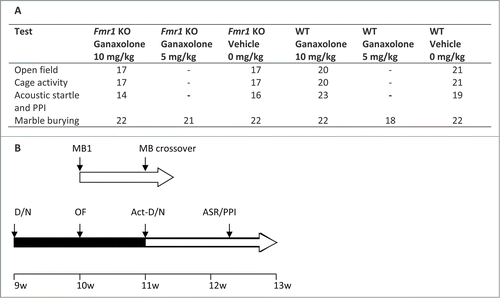
Figure 6. Schematic representation of test battery. (A) Overview of the behavioral assays and the number of animals in each experimental group. (B) Scheme of the order in which the behavioral assays were performed in group 1: marble burying (MB1); marble burying crossover (MB crossover); and group 2: reverse day/night cycle (D/N), open field (OF), cage activity (Act), reverse day/night cycle (D/N), acoustic startle response (ASR) and prepulse inhibition (PPI), as indicated above the horizontal arrows. The horizontal arrow is colored black when the animals are in a reversed day/night cycle. The inter-trial interval is indicated below the horizontal arrows and the age of the animals is indicated on the bottom line.
Sensory responses and sensorimotor gating were assessed by acoustic startle response and PPI (). Both fragile X patients and Fmr1 KO mice exhibit abnormalities in these tests, reflecting an altered sensitivity to sensory stimulation.Citation33 PPI was increased in vehicle-treated Fmr1 KO mice as compared to vehicle-treated WT controls (70 dB: p = 0.026, 75 dB: p = 0.075; 80 dB: p = 0.018; Tukey post hoc after one-way ANOVA). After ganaxolone treatment, we observed an intermediate effect on PPI in Fmr1 KO mice at 70 dB and 80 dB. More specifically, PPI was not significantly different from vehicle-treated Fmr1 KO mice, but it also did not differ from vehicle-treated WT mice (). We did not observe significant differences in acoustic startle response, neither between vehicle-treated Fmr1 KO mice and controls, or as a results of ganaxolone treatment ().
Discussion
As the fragile X syndrome is caused by loss-of-function of the RNA-binding protein FMRP, numerous studies have aimed to identify the mRNA targets of FMRP in order to elucidate part of the pathophysiological mechanisms of the disorder.Citation34 While hundreds of putative mRNA targets have been reported in recent large-scale screens,Citation9,14 only approximately a dozen have been biochemically validated to interact with FMRPCitation1, including mRNA of the GABAA receptor δ subunit.Citation6 These FMRP:mRNA interactions are of particular interest as aberrant GABAA receptor function is compatible with the phenotype of fragile X patients.Citation16
While decreased GABAA receptor mRNA expression has previously been detected in adult Fmr1 KO miceCitation19-21, we extended our earlier studies to young mice as deficits in GABAA receptor expression may vary during development.Citation24 We observed a significant reduction in α1, α2, α3, δ and γ2 mRNA in the Fmr1 KO cortex at P22. While α1, α3, δ and γ2 were also found reduced in adult cortex,Citation19 α2 expression was only reduced at young age. Surprisingly, we also detected significant reductions in the mRNA levels of all 3 α subunits that were measured in cerebellar samples. Although mRNA and protein expression studies in adult Fmr1 KO mice suggest that cerebellar GABAA receptors are unaffected,Citation19,22 a metabolomic study identified reduced GABA levels in the cerebellum at P12.Citation35 We confirmed reduced GABA concentrations in the cerebellum and detected a similar decrease in hippocampal samples, both at P22. Further research is required to determine whether these reductions are due to changes in intracellular or interstitial neurotransmitter levels, or both. Overall, our findings reveal that the expression of the GABAA receptor system of Fmr1 KO mice is affected in a brain region- and age-dependent manner.
Although molecular and electrophysiological GABAergic defects in the fragile X syndrome are well documented,Citation16,17 the causative mechanisms remain elusive. We were able to correct the underexpression of mRNAs of the α1 and δ subunits by reintroducing FMRP in Fmr1 KO mice. Interestingly, FMR1 is overexpressed in these YAC rescue mice, at both mRNA and protein level.Citation27 Despite the overexpression of FMRP, the level of GABAA receptor subunits did not exceed the level observed in controls. This is compatible with the hypothesis that FMRP does not influence transcription of the GABAA receptor subunits, but rather controls stability of its targets and prevents them from degrading by binding them in vivo.
In addition, we found that FMRP associates with mRNA encoding GABAA receptor subunits. More specifically, we have shown that such association is direct and entails the binding of the C-terminal part of FMRP with 3′UTRs of GABAA receptor δ subunit mRNA or the putative QGRS in the 3′UTR of α1 subunit mRNA. These findings are consistent with the previous antibody-positioned RNA amplification (APRA)-based identification of the GABAA receptor δ subunit mRNA as an FMRP target.Citation6 As the C-terminus of FMRP contains the RGG box, it is tempting to speculate that the in silico-predicted G-quadruplexes in α1 and δ mRNA are targeted by FMRP. However, the presence of a G-quadruplex was not experimentally observed in the mRNA of the δ subunit (H. Moine, personal communication). Thus, despite the direct binding to FMRP, the existence of a putative recognition sequence in the mRNA remains to be determined. In summary, our binding studies demonstrate that FMRP directly binds the mRNAs of at least 2 GABAA receptor subunits. These data, together with the evidence that increased FMRP levels in the YAC rescue mice do not mirror an increase in the mRNAs encoding α1 and δ subunits, suggest that FMRP might regulate the stability of these messengers. Absence of FMRP might thus result in an increased turnover of these mRNAs and, consequently, contribute to molecular and electrophysiological deficits in the GABAergic system.
Given the downregulation of several GABAA receptor subunits and the reduction in GABA levels in the Fmr1 KO mouse, we have examined if the administration of ganaxolone, a synthetic neurosteroid and positive allosteric modulator of GABAA receptors,Citation29,30 might have therapeutic potential. Neurosteroids are likely to affect both synaptic and extrasynaptic GABAA receptors, with δ-containing, extrasynaptic GABAA receptor subtypes being particularly sensitive to neurosteroid modulation.Citation36 After excluding sedative effects of acute ganaxolone treatment in 2 locomotor tests, open field and cage activity, we evaluated the effect of ganaxolone on specific behavioral characteristics of Fmr1 KO mice, i.e., increased burying in the marble burying assay Citation(ref.37, own observations), and altered sensory responses and sensorimotor gating in the acoustic startle response and PPI experiments.Citation32,38-40 The marble burying assay was used to evaluate the effect of ganaxolone on repetitive and perseverative behavior,Citation31 which relates to the autistic-like characteristics of fragile X patients.Citation32 Acute ganaxolone treatment led to a dose-dependent decrease of the burying behavior in Fmr1 KO mice, completely correcting the aberrant digging at the maximum dose tested (10 mg/kg). This effect was specific to Fmr1 KO mice, as behavior in WT mice was not affected by ganaxolone treatment. Sensory responses and sensorimotor gating were examined because they are affected in both fragile X patients and Fmr1 KO mice and could reflect sensory circuit defects contributing to the behavioral problems.Citation33 We observed a significant increase in PPI in Fmr1 KO mice and a trend toward decreased PPI after ganaxolone treatment. We did not observe any significant differences in acoustic startle response under the current experimental conditions, neither between vehicle-treated mice, nor after ganaxolone treatment. Therefore, changes in PPI were not confounded by baseline changes in acoustic startle response. Our findings, together with previous observations that ganaxolone completely prevented audiogenic seizures in Fmr1 KO mice,Citation41 show that acute administration of ganaxolone might have beneficial effects on some behavioral phenotypes in Fmr1 KO mice. Further studies are required to evaluate the effects of chronic ganaxolone treatment on Fmr1 KO mice. Of note, unlike the classical benzodiazepines, chronic neurosteroid treatment does not lead to tolerance, which makes them suitable for long-term use.Citation36
Conclusion
In summary, our analyses revealed that Fmr1 KO mice exhibit age- and brain region-dependent changes in GABAA receptor mRNA expression. Moreover, we detected direct interaction between FMRP and mRNA of the GABAA receptor subunits α1 and δ. Possible functional consequences of this binding remain to be determined. Additionally, acute treatment with the synthetic neurosteroid ganaxolone modulates specific behaviors of Fmr1 KO mice. Our results further encourage the use of this or other subtype-selective modulators as candidate drugs for the fragile X syndrome.
Materials and Methods
Mouse models
Housing conditions
All animals were housed in mixed genotype groups of approximately 5 littermates in standard mouse cages under conventional laboratory conditions (food and water ad libitum, constant room temperature and humidity, 12:12 h light-dark cycle). All experiments were carried out in compliance with the European Community Council directive (86/609/EEC) and approved by the Animal Ethics Committee of the University of Antwerp.
Fmr1 KO mice
Male Fmr1 knockout (KO) mice and wild-type (WT) littermates were generated by crossing females heterozygous for the Fmr1 mutation (B6.129P2-Fmr1tm1Cgr backcrossed for more than 20 generations to C57BL/6J) to C57BL/6J WT males (Charles River). Animals of 3 weeks of age were used for the expression and GABA ELISA experiments. The mice used for the pharmacological testing were 2-3 months of age when the experiments were initiated. Genotypes were determined by PCR on DNA isolated from tail biopsies.Citation42
YAC rescue mice
Male WT, Fmr1 KO and Fmr1 KO/YAC (YAC rescue) mice were obtained by backcrossing females heterozygous for the Fmr1 mutation (B6.129P2-Fmr1tm1Cgr backcrossed for more than 20 generations to C57BL/6J), to male WT/YAC transgenic mice (C57BL/6J background) (Fig. S1).Citation27 Animals of 10 weeks old were used for the expression experiments. After DNA isolation from mouse tails, genotyping was performed as previously described.Citation43
mRNA Expression experiments in Fmr1 KO mice and in YAC rescue mice
Fmr1 KO (n = 10) and WT littermates (n = 10) were sacrificed by cervical dislocation at the age of 3 weeks. Immediately after removing of the brain from the skull, the cerebellum, prefrontal cortex and hippocampus were isolated. The left and right sample of each anatomical region were separately snap-frozen in liquid nitrogen and stored at −80°C. One half of the samples was used for real-time PCR the other half for GABA ELISA (see below). Brain samples were homogenized using the Dispomix v1.4 homogenizer (Miltenyi Biotec, 130-093-235). Total RNA was then isolated using Trizol (Invitrogen - Life Technologies, 15596-026) according to the manufacturer's instructions. After removal of the remaining gDNA with DNA-Free (Ambion - Life Technologies, AM1906), the quality of the RNA samples was assessed with the Experion Automated Gel Electrophoresis System (Biorad, 701-7001). mRNA expression was analyzed using a 2-step real-time PCR assay. First, cDNA was obtained by reverse transcription of mRNA with the Transcriptor First Strand cDNA Synthesis kit (Roche Applied Science, 04379012001). First strand cDNA was subsequently used as a template for real-time PCR with the qPCR MasterMix Plus for SYBR Green 1 No Rox (Eurogentec, 05-SN2X-03+NR) and Lightcycler 480 thermocycler and detection platform (Roche Applied Science, 05015278001). We designed intron-flanking or intron-spanning primers (Integrated DNA technologies), for detection of GABAA receptor subunits (α1, α2, α3, β1, β2, γ1, γ2 and δ) and GABA metabolism enzymes (Abat, Gad1 and Gad2), to prevent amplification of gDNA (Primer Sequences, Supplementary Table S.1.). The specificity of the amplification was checked by performing melting curve analysis after PCR amplification (cycling conditions: 2 min 50°C; 10 min 95°C; 45 cycli with 15 s 95°C and 1 min 60°C; 5 s 95°C; 1 min 65°C; increase to 97°C at 11°C/s; 5 s 40°C). The obtained results were analyzed with qBase Plus software (Biogazelle). This analysis included quality control of the raw data, assessment of the reference gene stability and calculation of the normalization factors. More specifically, reference gene stability was assessed by 2 parameters; the gene stability value (M < 0.5) and coefficient of variation (CV < 25%) (Supplementary Table S.2.). mRNA expression levels were normalized by the geometric mean of the 3 most stably expressed reference genes selected from the geNormPlus Kit with Advanced Reference Genes (Primer Design, ge-SY-12). Then the relative expression (RE) was calculated, i.e. the ratio of the geometric mean of the normalized expression levels of the Fmr1 KO animals to that of the WT mice. Fmr1 expression was measured as a control. The real-time PCR experiments were repeated on a second batch of cDNA obtained from the original mRNA samples and similar results were obtained (data not shown). Statistical analysis of the quantitative real-time PCR data was done with the Mann-Whitney U non-parametrical test (IBM SPSS 20).
The relative residual expression levels of GABAA receptor subunits α1 and δ were determined in cortical samples of 10-week-old Fmr1 KO/YAC (YAC rescue) (n = 8) and Fmr1 KO (n = 10) mice as compared to WT (n = 10) as previously described,Citation19 using TaqMan probes (Applied biosystems, Supplementary Table S.3.). A Mann-Whitney U test was used to identify significant differences between Fmr1 KO or YAC rescue and WT mice, allowing comparison to our initial findings.
GABA ELISA
GABA-concentrations were determined in the cerebellum (n = 10/genotype) and hippocampus (n = 10/genotype) of Fmr1 KO and WT mice at postnatal day 22 using a GABA Research ELISA (LDN, BA E-2600). Tissue was homogenized in ice-cold Lysis Buffer (20 µl/mg RIPA Lysis and Extraction Buffer, 89900, and 1% Halt Protease Inhibitor Cocktail, 87786; Thermo Fisher Scientific) in the Dispomix v1.4 homogenizer (Miltenyi Biotec, 130-093-235). Lysates were centrifuged at 10,000 g for 10 min at 4°C. The supernatant was collected and the protein concentration was determined with the BCA Protein Assay (Thermo Fisher Scientific, PI-23225). Subsequently, 50 µg of total protein was used for the ELISA, dilutions were made in 1 mM HCl. The ELISA was performed according to the manufacturer's instructions, except for the washing step in the extraction phase (2 × 1 ml I-buffer) and the washing steps after the incubation with GABA Antiserum and Enzyme Conjugate (4 × 300 µl Wash Buffer). Statistical analysis was done with the Mann-Whitney U non-parametrical test (IBM SPSS 20).
Electrophoretic mobility shift assay
Direct protein:RNA binding was studied in electrophoretic mobility shift assays (EMSAs). We focused on the interaction of FMRP with G-rich sequences in mRNA.Citation10,11 The identification of putative quadruplex forming G-rich sequences (QGRS) in the 3' untranslated region (UTR) of the GABAA receptor α1 and δ subunits was done using the QGRS Mapper2 program (http://bioinformatics.ramapo.edu/QGRS2/index.php). G-quadruplexes are secondary RNA structures that consist of stacks of G-quartets or tetrads, with each quartet containing 4 guanines that interact via cyclic Hoogsteen hydrogen bonds.Citation2,44 Based on this in silico analysis, 6 constructs were selected: 3 constructs of GABAA receptor subunits, 2 positive controls and one negative (). The constructs were verified by sequencing and were then linearized using the manufacturer's protocol. The appropriate restriction enzymes are shown in . The linearized vectors were used as a template for the T7 or T3 polymerase to produce 32P-labeled RNAs in the presence of 50 μCi of [α−32P] UTP (Perkin Elmer, 3000 Ci/mmol), using the Riboprobe In Vitro Transcription System (Promega, P1430 and P1440). RNAs were purified using DNA-Free (Ambion - Life Technologies, AM1906) and Illustra NICK Columns (GE Healthcare, 17-0855-01).
The EMSAs were carried out using the FMRP C-terminal domain as the protein component for the assay, constructed and produced as previously described,Citation45,46 and conducted as reported previously.Citation5 Briefly, the reactions were performed in 150 mM KCl, 5 mM MgCl2, 2 mM DTT, 2% glycerol, 20 mM HEPES pH 7.5 and 500 ng of total yeast tRNA, incubating for 20 min at 25°C. The free RNAs and protein:RNA complexes were separated by electrophoresis on a 5% native polyacrylamide gel in 0.5 X Tris-borate-EDTA buffer at 4°C and then analyzed by autoradiography.
Pharmacological testing
Drug
The effects of the GABAA receptor positive allosteric modulator ganaxolone (a generous gift from Dr. Michael Rogawski, UC Davis, Sacramento, CA, USA) were evaluated in Fmr1 KO mice and WT littermates. Mice were randomly assigned to a treatment group and experiments were performed by a researcher blinded for the genotypes. Ganaxolone (10 mg/kg) or vehicle (22.5% 2-hydroxypropyl-β-cyclodextrin, Sigma-Aldrich, H107) was injected intraperitoneally, 10 min prior to testing. The dose was selected based on previous experiments in our group.Citation41 The injected volume was 10 ml/kg body weight.
Behavior experiments
The effects of ganaxolone were studied in adult mice using following behavioral assays: marble burying, open field activity, cage activity, acoustic startle and prepulse inhibition (PPI). In order to reduce the number of animals, some tests were performed consecutively with an interval of 7-9 days. gives a schematic representation of the test battery. The mice were 2-3 months old at the start of the experiments.
Open field activity
Overall exploratory activity and anxiety-related behavior were evaluated in the open field during the dark phase of the animals' activity cycle. Each mouse was placed in a brightly lit 50 cm × 50 cm arena for 10 min. The mice always began in the same corner and were given 1 min to adapt. A computerized video tracking system (Ethovision, Noldus) recorded the trajectories. Several parameters were assessed, including the total path length, path length in the periphery surrounding the center circle (i.e., total area minus center circle) and path length in the corners (7 cm × 7 cm), as well as the number of entries into the center circle and the corners of the open field. Significant differences in total distance were assessed using a one-way ANOVA, while statistical analysis of the other parameters was done using the Kruskall-Wallis test (n = 17-21 per group, ) (IBM SPSS 20).
Cage activity
Horizontal ambulatory cage activity was measured over a 1-h period in standard transparent mouse cages (22.5 cm × 16.7 cm × 14 cm; length × width x height, respectively) during the dark phase of the animals' activity cycle. The cages were placed between 3 infrared sensors (2 perpendicular to and one parallel to the length of the cage) in closed cabinets equipped with electrical ventilation fans to maintain the optimum temperature and lights to simulate the animals' 12:12h light-dark cycle. The number of beam interruptions was recorded using a microprocessor counter interfaced with a computer. The Kruskal-Wallis test was used for statistical analysis of the total activity count, measured as the number of beam crossings. Two-way repeated-measures ANOVA allowed to detect significant differences in genotype × time interaction (n = 17-21 per group, ) (IBM SPSS 20).
Marble burying
The marble burying assay was used to assess repetitive and perseverative behavior.Citation31 Twenty dark blue glass marbles (13 mm in diameter) were placed in a 4 × 5 matrix on top of 4-5 cm fresh bedding material (BF1, Bio-services-Ssniff) in a standard mouse cage (22.5 cm × 16.7 cm × 14 cm; length × width × height, respectively) with a filter top lid. Each mouse was allowed to explore the cage for 30 min. After carefully removing the mice, the number of marbles buried (>2/3 covered by bedding material) was evaluated. The marble burying test was not included in the series of consecutive behavioral tests (), but was performed separately with a crossover design and a 7 day inter-trial interval in 2 independent experiments (10 mg/kg ganaxolone versus 0 mg/kg and 5 mg/kg ganaxolone vs. 0 mg/kg). Mice were tested between 07.00 a.m. and 11.00 a.m. under standard lighting conditions. Data were analyzed using a 2-way ANOVA (with genotype x treatment interaction) with Tukey post hoc test. A separate independent-samples T-test was used to compare Fmr1 KO and WT mice at 0 mg/kg dose (n = 18-22/group; ) (IBM SPSS 20).
Acoustic startle and prepulse inhibition
The sensory response and sensorimotor gating were quantified as acoustic startle response and PPI of startle as previously described Citation43 with minor adaptations. Mice were placed in a plexiglas restrainer mounted upon a motion-sensitive plate within the sound-attenuating acoustic startle box (Kinder Scientific). They were allowed to habituate for 5 min, in the presence of background noise (65 dB, white noise). Following habituation, the response of the mice was measured in 8 different trial types. During the first trial type no stimulus was presented, which allowed the measurement of baseline movement. The second trial type consisted of a startle stimulus of 120 dB for 40 ms. In the other trial types, 3 different prepulse stimuli (70 dB, 75 dB and 80 dB; for 20 ms) were presented alone or 100 ms prior to the startle stimulus to assess PPI of the startle response. Each trial type was presented 10 times, once per block of 8 trials in a pseudorandom order. The inter-trial interval was 10 to 20 s. Then, the quality of the data was evaluated. More specifically, we confirmed that the startle response was significantly different from baseline movement and that the prepulses alone did not elicit a startle response, in other words that the prepulse response was not significantly different from the baseline movement. Subsequently, the maximum startle amplitude following each type of stimulus was recorded, averaged across the 10 trials for each mouse and used as a dependent variable. The percentage of PPI was calculated as 100 - [(response to trials with prepulse and startle stimulus/response to trials with startle stimulus alone) x 100]. The experiment was performed between 1.00 p.m. and 6.00 p.m. Statistical analysis was performed using a 1-way ANOVA with Tukey post hoc test (n = 14-23 per group; ) (IBM SPSS 20).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
989114__SUPPLEMENTARY_MATERIAL.pdf
Download PDF (171.4 KB)Acknowledgment
The plasmid containing the N19 fragment of FMR1 was a kind gift from Dr. Hervé. Moine (IGBMC, Strasbourg, France). We thank Dr. Hervé Moine and Dr. Marie-Cécile Didiot for the work on the G-quartet in the δ subunit message. Ganaxolone was a kind gift from Dr. Michael Rogawski (UC Davis, Sacramento, CA, USA). Furthermore, we thank Dr. Stefanie Dedeurwaerdere and Dr. Paul Van de Heyning (University of Antwerp, Antwerp, Belgium) for the use of the Acoustic Startle apparatus and Dr. Zsuzsanna Callaerts-Vegh (KU Leuven, Leuven, Belgium) for her advice on the marble burying and the acoustic startle and PPI experiments.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
This study was supported through grants of the Fragile X Research Foundation (FRAXA), Research Foundation-Flanders (FWO-Flanders), Fondation Jérôme Lejeune, Interuniversity Poles of Attraction of the Belgian Federal Science Policy Office (P7/16), Methusalem Excellence Grant of the Flemish Government, agreement between Institute Born-Bunge and the University of Antwerp, the Medical Research Foundation Antwerp, Neurosearch Antwerp, the Thomas Riellaerts Research Fund, VIB (Flemish Institute for Biotechnology) and Associazione Italiana Sindrome Fragile X. S.B. has a PhD grant from the Agency for Innovation by Science and Technology (IWT) and D.V.D. is a Postdoctoral Fellow of the FWO-Flanders.
References
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol 2012; 7:219–45; PMID:22017584; http://dx.doi.org/10.1146/annurev-pathol-011811-132457
- Maurin T, Zongaro S, Bardoni B. Fragile X Syndrome: From molecular pathology to therapy. Neurosci Biobehav Rev 2014; 46 Pt 2:242–55; PMID:24462888; http://dx.doi.org/10.1016/j.neubiorev.2014.01.006
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 2008; 60:201–14; PMID:18957214; http://dx.doi.org/10.1016/j.neuron.2008.10.004
- Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest 2012; 122:4314–22; PMID:23202739; http://dx.doi.org/10.1172/JCI63141
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SGN, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci 2007; 10:578–87; PMID:17417632; http://dx.doi.org/10.1038/nn1893
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 2003; 37:417–31; PMID:12575950; http://dx.doi.org/10.1016/S0896-6273(03)00034-5
- Fernandez E, Rajan N, Bagni C. The FMRP regulon: from targets to disease convergence. Front Neurosci 2013; 7:191; PMID:24167470; http://dx.doi.org/10.3389/fnins.2013.00191
- Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Gen Dev 2005; 19:903–18; PMID:15805463; http://dx.doi.org/10.1101/gad.1276805
- Ascano M, Jr., Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 2012; 492:382–6; PMID:23235829; http://dx.doi.org/10.1038/nature11737
- Darnell J, Jensen K, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 2001; 107:489–99; PMID:11719189; http://dx.doi.org/10.1016/S0092-8674(01)00566-9
- Schaeffer C, Bardoni B, Mandel J-L, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J 2001; 20:4803–13; PMID:11532944; http://dx.doi.org/10.1093/emboj/20.17.4803
- Bechara EG, Didiot MC, Melko M, Davidovic L, Bensaid M, Martin P, Castets M, Pognonec P, Khandjian EW, Moine H, et al. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol 2009; 7:e16; PMID:19166269; http://dx.doi.org/10.1371/journal.pbio.1000016
- Chen L, Yun SW, Seto J, Liu W, Toth M. The fragile X mental retardation protein binds and regulates a novel class of mRNAs containing U rich target sequences. Neuroscience 2003; 120:1005–17; PMID:12927206; http://dx.doi.org/10.1016/S0306-4522(03)00406-8
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP Stalls Ribosomal Translocation on mRNAs Linked to Synaptic Function and Autism. Cell 2011; 146:247–61; PMID:21784246; http://dx.doi.org/10.1016/j.cell.2011.06.013
- Braat S, Kooy RF. Fragile X syndrome neurobiology translates into rational therapy. Drug Discov Today 2014; 19(4):510–9
- D'Hulst C, Kooy RF. The GABA(A) receptor: a novel target for treatment of fragile X? Trends Neurosci 2007; 30:425–31; PMID:17590448; http://dx.doi.org/10.1016/j.tins.2007.06.003
- Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev Neurosci 2011; 33(5):349–64; PMID:21934270; http://dx.doi.org/10.1159/000329420
- Braat S, Kooy RF. Insights into GABAergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology 2015; 88:48-54; PMID:25016041; http://dx.doi.org/10.1016/j.neuropharm.2014.06.028
- D'Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 2006; 1121:238–45; PMID:17046729; http://dx.doi.org/10.1016/j.brainres.2006.08.115
- D'Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS). Brain Res 2009; 1253:176–83; PMID:19070606; http://dx.doi.org/10.1016/j.brainres.2008.11.075
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D'Hooge R, Severijnen L-A, Willemsen R, Tassone F, Kooy RF. Expression profiling reveals involvement of the GABAA receptor subunit  in the fragile X syndrome. Neurobiol Dis 2006; 21:346–57; PMID:16199166; http://dx.doi.org/10.1016/j.nbd.2005.07.017
- El Idrissi A, Ding X-H, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci Lett 2005; 377:141–6; PMID:15755515; http://dx.doi.org/10.1016/j.neulet.2004.11.087
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of Tonic GABAergic Inhibition in a Mouse Model of Fragile X Syndrome. Cereb cortex 2009; 19:1515–20; PMID:18787232; http://dx.doi.org/10.1093/cercor/bhn159
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early Developmental Alterations in GABAergic Protein Expression in Fragile X Knockout Mice. Neuropharmacology 2010; 59(3):167–71; PMID:20470805; http://dx.doi.org/10.1016/j.neuropharm.2010.05.002
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Gen Biol 2002; 3:34.1-.11; http://dx.doi.org/10.1186/gb-2002-3-7-research0034
- Mientjes EJ, Nieuwenhuizen I, Kirkpatrick L, Zu T, Hoogeveen-Westerveld M, Severijnen L, Rifé M, Willemsen R, Nelson DL, Oostra BA. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis 2006; 21:549–55; PMID:16257225; http://dx.doi.org/10.1016/j.nbd.2005.08.019
- Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet 2000; 9:1145–59; PMID:10767339; http://dx.doi.org/10.1093/hmg/9.8.1145
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 1996; 19:139–43; PMID:8658597; http://dx.doi.org/10.1016/S0166-2236(96)80023-3
- Nohria V, Giller E. Ganaxolone. Neurotherapeutics 2007; 4:102–5; PMID:17199022; http://dx.doi.org/10.1016/j.nurt.2006.11.003
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther 1997; 280:1284–95; PMID:9067315
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009; 204:361–73; PMID:19189082; http://dx.doi.org/10.1007/s00213-009-1466-y
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res 2011; 4:40–56; PMID:21268289; http://dx.doi.org/10.1002/aur.168
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry 2004; 9:417–25; PMID:14981523; http://dx.doi.org/10.1038/sj.mp.4001432
- Suhl JA, Chopra P, Anderson BR, Bassell GJ, Warren ST. Analysis of FMRP mRNA target datasets reveals highly associated mRNAs mediated by G-quadruplex structures formed via clustered WGGA sequences. Hum Mol Genet 2014; 23(20):5479–91; PMID:24876161; http://dx.doi.org/10.1093/hmg/ddu272
- Davidovic L, Navratil V, Bonaccorso CM, Catania MV, Bardoni B, Dumas ME. A metabolomic and systems biology perspective on the brain of the Fragile X syndrome mouse model. Gen Res 2011; PMID:21900387
- Reddy DS, Rogawski MA. Neurosteroids - Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, eds. Jasper's Basic Mechanisms of the Epilepsies. Bethesda (MD), 2012.
- Veeraragavan S, Graham D, Bui N, Yuva-Paylor LA, Wess J, Paylor R. Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS). Behav Brain Res 2012; 228:1–8; PMID:22123412; http://dx.doi.org/10.1016/j.bbr.2011.11.018
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 2001; 103:1043–50; PMID:11301211; http://dx.doi.org/10.1016/S0306-4522(01)00036-7
- Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res 2002; 927:8–17; PMID:11814427; http://dx.doi.org/10.1016/S0006-8993(01)03309-1
- Errijgers V, Fransen E, D'Hooge R, De Deyn PP, Kooy RF. Effect of genetic background on acoustic startle response in fragile X knockout mice. Genet Res 2008; 90:341–5; http://dx.doi.org/10.1017/S0016672308009415
- Heulens I, D'Hulst C, Van Dam D, De Deyn PP, Kooy RF. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav Brain Res 2012; 229:244–9; PMID:22285772; http://dx.doi.org/10.1016/j.bbr.2012.01.031
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 1994; 78:23–33; PMID:8033209
- Paylor R, Yuva-Paylor LA, Nelson DL, Spencer CM. Reversal of sensorimotor gating abnormalities in Fmr1 knockout mice carrying a human Fmr1 transgene. Behav Neurosci 2008; 122:1371–7; PMID:19045956; http://dx.doi.org/10.1037/a0013047
- Halder K, Hartig JS. RNA quadruplexes. Met Ions life Sci 2011; 9:125–39; PMID:22010270; http://dx.doi.org/10.1039/9781849732512-00125
- Adinolfi S, Bagni C, Musco G, Gibson T, Mazzarella L, Pastore A. Dissecting FMR1, the protein responsible for fragile X syndrome, in its structural and functional domains. Rna 1999; 5:1248–58; PMID:10496225; http://dx.doi.org/10.1017/S1355838299990647
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 2003; 112:317–27; PMID:12581522; http://dx.doi.org/10.1016/S0092-8674(03)00079-5
