 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Like most basic molecular mechanisms, programmed –1 ribosomal frameshifting (−1 PRF) was first identified in viruses. Early observations that global dysregulation of −1 PRF had deleterious effects on yeast cell growth suggested that −1 PRF may be used to control cellular gene expression, and the cell cycle in particular. Collection of sufficient numbers of viral −1 PRF signals coupled with advances in computer sciences enabled 2 complementary computational approaches to identify −1 PRF signals in free living organisms. The unexpected observation that almost all −1 PRF events on eukaryotic mRNAs direct ribosomes to premature termination codons engendered the hypothesis that −1 PRF signals post-transcriptionally regulate gene expression by functioning as mRNA destabilizing elements. Emerging research suggests that some human diseases are associated with global defects in −1 PRF. The recent discovery of −1 PRF signal-specific trans-acting regulators may provide insight into novel therapeutic strategies aimed at treating diseases caused by changes in gene expression patterns.
Introduction
Cells regulate gene expression via diverse mechanisms. From mRNA transcription to protein degradation, many regulatory systems affect the timing, localization, and rate of each reaction. Gene expression is primarily concerned with the abundance and translational activity of mRNA; therefore expression is increased when a message is transcribed more rapidly, stabilized by the cell, or more available to actively translating ribosomes. Increased mRNA degradation, decreased transcription, translational silencing, and the storage of mRNA are the hallmarks of decreased expression.
The center of this spectrum between mRNA synthesis and protein degradation is translation, the timely and high-fidelity process of bringing together mRNA, aminoacylated- tRNA, and the ribosome. Cellular functions are generally not impacted by sense errors, but nonsense, missense, and errors which change the reading frame tend to be deleterious; the translational apparatus has evolved to ensure that these are extremely rare. Simultaneously, the complex interplay of factors which effect translation suggest that the ribosome is a likely post-transcriptional regulation nexus of gene expression. Most evidence gathered thus far concentrates primarily on cis-acting elements in the 5′ and 3′ untranslated regions (UTRs) of mRNAs, and the trans-acting factors with which they interact. Coding sequences have more recently been examined for effects on post-transcriptional control, and multiple cis-acting mRNA elements have been found which cause elongating ribosomes to recode the mRNA sequence.Citation1-4 In all kingdoms of life, these elements include, but are not limited to, sequences responsible for +1 and −1 programmed ribosomal frameshifting, termination codon suppression, stop-start elements, selenocysteine incorporation, and in archaea, pyrolysine incorporation.
Reading frame definition and maintenance
Translational reading frame maintenance is a primary determinant of the proteome. If the specificity of this process is lost or degraded, cells will almost certainly die. This is a central point of attack by many antibiotics, which capitalize on small but distinct differences between bacterial and eukaryotic ribosomes.Citation5,6 Reading frame is determined by recognition of a start codon by an initiator tRNA in the ribosome's decoding center and is maintained through the consistent translocation by 3 nucleotides during elongation. The three stop codons do not correspond to any tRNAs, this enables the release factor complex to initiate translation termination. Translational recoding comprises the spectrum of events which occur when canonical translation is disrupted. This ranges from mechanisms which subvert initiation,Citation7 alter the equilibria of events during elongation,Citation8 and affect the termination processCitation9 generally resulting in suppression or reinitiation. This document mainly focuses on a particular recoding event called programmed −1 ribosomal frameshifting that occurs during elongation.
Programmed −1 Ribosomal Frameshifting
Programmed −1 ribosomal frameshifting (PRF) is a recoding mechanism historically associated with virusesCitation10 and retrotransposons.Citation11,12 This is due at least in part to the much smaller and compact nature of viral genomes and the relative simplicity in searching their kilobase scale genomes as opposed to megabase bacterial genomes or gigabase eukaryotic genomes. When the gag amber termination of the Rous sarcoma virus was found to be bypassed in favor of translation of a downstream −1 reading frame, 2 relatively simple and testable possibilities were considered: either a splicing event or the programmed shift of ribosomes into the new reading frame.Citation10 Sequencing of the relevant region of the genome and in-vitro transcription/translation experiments supported the latter hypothesis and suggested that ‘shifty tRNAs’ caused the actual frameshift events. Shortly thereafter, reports described new RNA structures called pseudoknots, which when present in viral mRNAs could either repress translationCitation13 or stimulate efficient ribosomal frameshifting.Citation14 The Recode databasesCitation15,16 (http://recode.ucc.ie) continue to categorize and describe these viral signals.
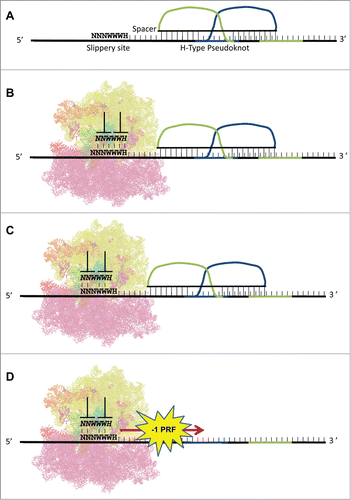
A PRF signal is defined as a cis-acting mRNA element that stochastically redirects translating ribosomes into an alternate reading frame. The most well characterized −1 PRF signals follow a relatively consistent pattern of stimulatory element proximally downstream of a group of weakly pairing bases (the “slippery heptamer”) comprised of Nx NxNxWy WyWyHz; the spaces delineate the incoming reading frame, NWH refer to the IUPAC definitions, and xyz denote identical nucleotides. There is some debate regarding the relative contributions of the slippery heptamer, downstream stimulatory element, and the space between them; but all models of −1 PRF agree that the downstream mRNA structure causes elongating ribosomes to pause while tRNAs are positioned over the slippery site. The nature of the codon:anticodon interactions at the slippery site facilitates slippage of a fraction of paused ribosomes backward (5′) by one base through pairing at the non-wobble positions. The weak homopolymer nucleotide sequences (poly-U or poly-A) are of particular interest in −1 PRF, as they are often coupled with pauses in translation in order to effect the frameshifting event.Citation17 H-type mRNA pseudoknots are the most common stimulatory structure, but other structures, including proteins bound to stem-loops,Citation18 variously sized stem-loops,Citation19 and RNA triplexesCitation20 can also promote efficient frameshifting. The general mechanism and structure of a typical −1 PRF signal is diagrammed in .
Figure 1. −1 PRF signals: structure and mechanism. (A) A typical −1 PRF signal is composed of 3 elements. 1) a heptameric “slippery site," 2) a short spacer, and 3) a stable mRNA structure, e.g., an H-type pseudoknot. (B) The pseudoknot forces an elongating ribosome to pause with its A- and P-site tRNAs positioned at the slippery site in the 0-frame. (C) Slippage of the tRNAs by one base in the 5’ (−1) direction enables non-wobble base pairing. (D) The ribosome denatures the pseudoknot, and translation elongation resumes in the −1 reading frame.
Genomic −1 PRF
Examination of the history of modern molecular genetics shows that most basic molecular mechanisms were first observed in viruses. Therefore, it was reasonable to hypothesize that −1 PRF is also used to control expression of cellular genes. Indeed, the serendipitous discoveries of −1 PRF signals of viral origin in the mammalian PEG10Citation21,22 and Edr1Citation23 mRNAs suggested that more of these elements were hidden in eukaryotic genomes. Simultaneously, genetic studies in yeast suggested that −1 PRF may play a role in cell cycle control.Citation24 Thus began the search for −1 PRF signals in eukaryotic genomes.
There are 2 complementary approaches when searching genome databases for potential frameshift signals. The first is to search for genes harboring conserved overlapping open reading frames.Citation25 This approach has been highly successful in identifying new viral −1 PRF signals, and conserved +1 PRF signals in ornithine decarboxylase genes.Citation26 However, while this enables identification of novel PRF signals, it enforces the assumption that frameshifting results in a new functional protein. The second method is to search for sequence motifs that conform to known PRF signals. Though limited to one class of signal, this method does not assume that frameshifting results in a C-terminal extension product.
The efforts of our laboratory have focused on this latter approach. In particular, −1 PRF was chosen because there are sufficient viral examples to enable the generation of heuristics. This is in contrast to +1 frameshifting, which appears to be idiosyncratic. The basic strategy is to perform a pattern-match based search for allowable heptameric slippery sequences followed by strong downstream structure; this search is NP-completeCitation27 and therefore computationally difficult. The initial study using this approach pressed the available CPU limits at the time, taking significant time for relatively small numbers of sequences.Citation28 Later searches against the yeast genome refined this strategy and used significantly greater computational resources, allowing the yeast genome to be exhaustively searched in months.Citation29 Further refinements, increases in memory/CPU, and larger computational resources made it possible to complete approximately one genome per weekCitation30 along with comparisons against randomized sequences. Analysis of multiple genomes reveals that approximately 10% of annotated genes contain at least one high-confidence potential frameshift signal. A searchable database of predicted eukaryotic −1 PRF signals is available in the Predicted Ribosomal Frameshift Database (PRFdb, www.prfdb.umd.edu).Citation30
An unexpected result: cells use −1 PRF to control mRNA abundance
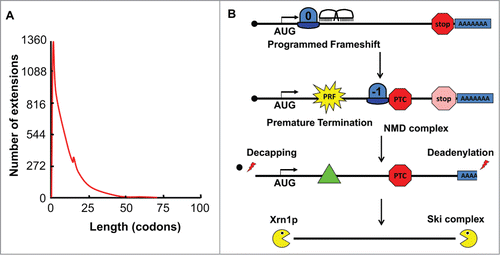
As noted above, a motif based search removes the assumption that frameshift events produce functional extended peptides. This led to the surprising observation that only 0.07% candidate −1 PRF containing sequences extend more than 30 codons beyond the frameshift event, regardless of the cellular genome examined (). The observation that the vast majority of frameshift events direct translating ribosomes to premature termination codons engendered the hypothesis that −1 frameshift signals function as mRNA destabilizing elements through the nonsense mediated mRNA decay pathway (NMD)Citation31 (). This was initially validated in yeast cells using a well-defined viral frameshift signal and further analysis suggested a role for No-Go decay (NGD) as well.Citation32 More recent studies in our laboratory demonstrate that this general rule is also true in human cells.Citation33
Figure 2. −1 PRF signals function as mRNA destabilizing elements. (A) Data from the programmed −1 ribosomal frameshift database (prfdb.umd.edu) plotting the number of −1 frame encoded C-terminal extensions (y-axis) versus their lengths in codons (x-axis) reveals that >99% of −1 PRF events direct ribosomes to termination codons within 30 codons. (B) Model: a −1 PRF event directs a ribosome to a premature termination codon. This triggers recruitment of the Nonsense Mediated mRNA Decay (NMD) complex to the mRNA, clearing the ribosome and initiating deadenylation of the 3’ end followed by decapping of the 5’ end. . The mRNA then becomes a substrate for exonucleolytic degradation.
−1 PRF and gene expression
If ∼10% of cellular mRNAs are controlled by −1 PRF, what is the biological significance of this phenomenon? As noted above, the first hint that −1 PRF may have a physiological role came from the observation that mutants that promote global increased rates of −1 PRF appeared to disrupt the cell cycle in yeast.Citation34 Indeed, a general observation in our laboratory over the past 2 decades has been that mutations that globally alter rates of −1 PRF compromise cell growth and viability.Citation35
The serendipitous discovery of a +1 PRF signal in the yeast EST3 mRNA, which encodes a component of telomerase,Citation36 prompted a search the PRFdb for −1 PRF signals in additional mRNAs encoding proteins involved in telomere maintenance. Operational −1 PRF signals (defined as promoting ≥1% frameshifting) were identified in 4 mRNAs encoding proteins critical for yeast telomere maintenance. These are: EST2, encoding the reverse transcriptase component of telomerase; EST1, encoding the protein that “docks” telomerase to chromosome ends; and STN1 and CDC13 mRNAs, which encode proteins involved in recruiting telomerase to shortened telomeres. These were all shown to function as NMD-dependent mRNA destabilizing elements. The wide range of −1 PRF efficiencies (from 2% - 70%) promoted by these elements enabled characterization of a simple exponential decay function between −1 PRF efficiency and mRNA destabilizing activity:
where x denotes PRF efficiency and mRNA abundance is a function of x. Abrogation of −1 PRF in the EST2 mRNA (leading to increased expression of this protein) yielded cells with telomeres of intermediate length, consistent with prior studies showing similar effects upon overexpression of this gene, or STN1 or CDC13.Citation37-40 Importantly, abrogation of NMD resulted in cells with very short telomeres, consistent with NMD being epistatic to −1 PRF. Microscopic examination of these cells revealed that a large fraction were arrested at the G2/M boundary characterized by large mother cells attached to equally large daughter cells. Indeed, many of these cells had additional buds , characteristic of cell cycle “escape” mutants. In unpublished studies, operational frameshift signals have been identified in at least 2 human messages encoding proteins required for telomere maintenance; suggesting that −1 PRF may also play a role in human telomere maintenance and aging.
Model: how −1 PRF may control telomere length
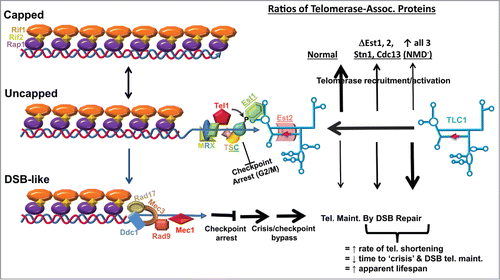
In yeast, telomerase abundance is strictly limited: it is estimated that diploid cells, which contain 64 chromosome ends, only contain ∼29 telomerase molecules,Citation41 consistent with observations that limitation of telomerase levels is required for telomere length homeostasis.Citation42 Current modelsCitation43,44 posit that telomeres exist in a range of states, from long, and fully capped (by Rap1p, Rif2p, Rif1p trimers) to short and uncapped, and that the small fraction of short uncapped telomeres present in any given cell cycle are preferentially repaired. As shown in , as telomeres age, they progressively shorten, and at some point reach an intermediate, uncapped status. This recruits a complex containing the MRX complex (Mre11p, Rad50p, Xrs2p) plus Tel1p, which in turn recruits the CST complex (Cdc13p, Stn1p and Ten1), inducing checkpoint arrest at the G2/M boundary. Phosphorylation of Cdc13p by Tel1p enables recruitment of telomerase through Est1p, stimulating telomere repair, and releasing cells from checkpoint arrest. Failure to recruit telomerase leads to further telomere shortening, where they eventually resemble double-stranded breaks (DSB). These short telomeres recruit the DSB repair machinery, resulting in strong checkpoint arrest at the G2/M boundary. Eventually, these short telomeres are maintained by this machinery, resulting in telomere end joining, and bypass of checkpoint arrest, i.e. multiply budded cells. The net effect is to “immortalize” telomeres, resulting in longer lifespans, but at the cost of genome integrity. As shown in , we propose that −1 PRF is used to maintain the correct stoichiometric ratios of telomerase components critical for telomerase recruitment. Changes in the expression of any one of these components, e.g. by abrogating −1 PRF in EST2 or overexpressing any single component, has dominant-negative effects on telomerase recruitment resulting in the observed increased rates of telomere shortening, consistent with the intermediate telomere lengths observed in these mutants. We further propose that changing the expression of all of the −1 PRF containing mRNAs, e.g., by globally changing rates of −1 PRF or by inactivation of NMD, has an even more dramatic effect, accounting for the very short telomeres observed in these classes of mutants.
Figure 3. Model: telomerase recruitment to uncapped telomeres is controlled by the relative stoichiometries of telomerase components in yeast. Top left depicts a fully capped telomere. As telomeres shorten, they become uncapped (middle left), recruiting the MRX-Tel1p complex, which in turn recruits the CST complex to the telomere end. Phosphorylation of Cdc13p by Tel1p recruits telomerase via Est1p. Est2p is the reverse transcriptase component of telomerase. If telomeres continue to shorten, they resemble chromosomes with double stranded breaks (DSB-like, lower left), recruiting DNA repair machinery. This results in cell growth arrest at checkpoint (G2/M). If cells cannot repair the defect, they undergo “crisis” and a subpopulation will bypass arrest, maintaining their chromosome ends by DSB repair. Operational −1 PRF signals have been identified in the STN1, CDC13, EST1 and EST2 mRNAs. We propose that their relative abundances are controlled by −1 PRF. In optimal conditions, precisely controlled rates of −1 PRF ensures that these proteins are present in the correct stoichiometries, maximizing telomere repair (bold up arrow), and minimizing progression to the DSB-like state. When expression of any one of these genes is altered, e.g. telomerase recruitment is less efficient and more telomeres progress more rapidly to the DSB-like state. When expression of all 4 are altered, e.g., by global changes in −1 PRF or by abrogation of NMD, telomeres progress rapidly to the DSB-like state.
Regulation of −1 PRF
If −1 PRF is normally employed to control gene expression, it stands to reason that −1 PRF itself should be subject to regulation. As suggested above, since global changes in −1 PRF tend to be detrimental to cells, regulation of −1 PRF should be sequence-specific. Given that −1 PRF is directed by cis-acting elements in mRNAs, sequence-specific interactions could be mediated by either base-pairing interactions with trans-acting RNAs, or by highly specific interactions with trans-acting proteins. Indeed, examples of both cases have been recently documented. The human CCR5 mRNA harbors a −1 PRF signal that functions as an mRNA destabilizing element by directing elongating ribosomes to premature termination codons, and we recently demonstrated that sequence specific interactions between this element and at least 2 micro-RNAs (miRNAs) promote increased rates of -1 PRF.Citation33 Mapping of the miRNA/−1 PRF signal interaction suggested that formation of an RNA-triplex structure stabilizes the frameshift-stimulating mRNA pseudoknot, leading to increased ribosome pause times at the slippery sequence, further enhancing frameshifting. With this in mind, it is also possible that trans-acting RNAs that destabilize −1 PRF stimulating pseudoknots may also exist, i.e., these would have −1 PRF inhibitory activity. Trans-acting proteins can also stimulate −1 PRF: in the porcine reproductive and respiratory syndrome virus (PRRSV), both −1 and −2 PRF are stimulated by the virus-encoded nsp1β replicase subunit that specifically interacts with sequence containing the slippery site.Citation45
Emerging evidence for a role of −1 PRF in human disease
To date, no direct connection has been established between changes in −1 PRF and human disease. However, a growing body of evidence suggests that such linkages may be forthcoming. The DKC1 gene encodes dyskerin, the protein that catalyzes conversion of uridines into pseudouridine in ribosomal rRNAs. Patients harboring mutations in this gene present with X-linked dyskeratosis (X-DC), a congenital disease characterized by bone marrow failure, dystrophic nails, mucosal leukoplakia, mottled rashes, congenital anomoalies and additional clinical presentations.Citation46 Hypo-pseudouridulated yeast and human ribosomes have lower affinities for tRNAs, resulting in greater rates of tRNA slippage at −1 PRF signals.Citation47 In unpublished work, we have shown that these mutant yeast cells have shortened telomeres, consistent with the progeria like symptoms of this disease. Indeed, X-DC is a member of a general class of diseases called ribosomopathies, which are caused by mutations in ribosomal protein genes and genes involved in ribosome biogenesis.Citation48 Unpublished yeast based studies in our laboratory suggest that translational fidelity defects including altered rates of −1 PRF, may play important roles in this general class of diseases. Spinocerebellar ataxia 26 (SCA26) is caused by a mutation in eukaryotic translation elongation factor 2 (eEF2), the GTPase that translocates ribosomes along mRNAs.Citation49 −1 PRF can occur during translocation, and inhibition of this process can stimulate this.Citation50 −1 PRF is elevated In yeast cells expressing mutant forms of eEF2 harboring the SCA26-equivalent mutation,Citation49 and in unpublished work, we have observed that this also occurs in cells derived from SCA26 patients. The finding that the same mutation in ribosomal protein L10 (eL16) found in a significant fraction of patients with T-cell lymphoblastic leukemia (T-ALL) also promotes increased rates of −1 PRF (by promoting decreased ribosomal affinity for aminoacyl-tRNA) provides evidence that somatically acquired mutations that affect −1 PRF may contribute to at least some cancers.Citation51
Summary and Perspectives
Like many basic molecular regulatory mechanisms, while −1 PRF was discovered in viruses it has now been found to be involved in the expression of a significant number of eukaryotic genes. Surprisingly, unlike viruses, where −1 PRF is used to expand the genomic coding content, it appears that −1 PRF signals are used to post-transcriptionally regulate gene expression by functioning as mRNA destabilizing elements. Emerging evidence links global defects in −1 PRF to a growing number of human diseases. Recent studies in yeast revealed the importance of this mechanism in telomere maintenance and cell-cycle control, and current research suggests that may also be applicable to human cells. The recent finding of −1 PRF signal-specific regulation by miRNAs has solved one of the central questions in the field, and in combination with the telomerase studies, provokes the hypothesis that aging may be programmed in part by −1 PRF. The ability of trans-acting factors to manipulate −1 PRF also suggests therapeutic approaches including recombinant proteins, and synthetic non-coding RNAs/RNA analogs. Other more global approaches may include use of small molecule modulators of −1 PRF, or targeting of downstream pathways, e.g. nonsense mediated mRNA decay. The new paradigms described here will continue to guide a diverse set of research efforts into the future.
Acknowledgments
We would like to thank members of the Dinman laboratory, both past and present, who contributed to this research.
Funding
This work was supported in part by a grant to JDD by the National Institutes of Health, R01 HL119439.
References
- Dinman JD. Mechanisms and implications of programmed translational frameshifting. Wiley InterdiscipRevRNA 2012; 3:661-73; PMID:22715123; http://dx.doi.org/10.1002/wrna.1126
- Antonov I, Coakley A, Atkins JF, Baranov P V, Borodovsky M. Identification of the nature of reading frame transitions observed in prokaryotic genomes. Nucleic Acids Res 2013; 41:6514-30; PMID:23649834; http://dx.doi.org/10.1093/nar/gkt274
- Namy O, Rousset JP, Napthine S, Brierley I. Reprogrammed genetic decoding in cellular gene expression. MolCell 2004; 13:157-68; PMID:14759362
- Urbonavicius J, Stahl G, Durand JM, Ben Salem SN, Qian Q, Farabaugh PJ, Bjork GR. Transfer RNA modifications that alter +1 frameshifting in general fail to affect −1 frameshifting. RNA 2003; 9:760-8; PMID:12756333; http://dx.doi.org/10.1261/rna.5210803
- Steitz TA. On the structural basis of peptide-bond formation and antibiotic resistance from atomic structures of the large ribosomal subunit. FEBS Lett 2005; 579:955-8; PMID:15680981; http://dx.doi.org/10.1016/j.febslet.2004.11.053
- Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 2000; 407:340-8; PMID:11014183; http://dx.doi.org/10.1038/35030019
- Pestova T V, Kolupaeva VG, Lomakin IB, Pilipenko E V, Shatsky IN, Agol VI, Hellen CU. Molecular mechanisms of translation initiation in eukaryotes. Proc Nat lAcad Sci USA 2001; 98:7029-36.
- Sulima SO, Gülay SP, Anjos M, Patchett S, Meskauskas A, Johnson AW, Dinman JD. Eukaryotic rpL10 drives ribosomal rotation. Nucleic Acids Res 2014; 42:2049-63; PMID:24214990; http://dx.doi.org/10.1093/nar/gkt1107
- Bertram G, Innes S, Minella O, Richardson JP, Stansfield I. Endless possibilities: translation termination and stop codon recognition. Microbiology-Uk 2001; 147:255-69; PMID:11158343
- Jacks T, Varmus HE. Expression of the Rous Sarcoma Virus pol gene by ribosomal frameshifting. Science 1985; 230:1237-42; PMID:2416054; http://dx.doi.org/10.1126/science.2416054
- Craigen WJ, Cook RG, Tate WP, Caskey CT. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci USA 1985; 82:3616-20; PMID:3889910
- Clare JJ, Belcourt M, Farabaugh PJ. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc Nat lAcad Sc iUSA 1988; 85:6816-20; PMID:2842793; http://dx.doi.org/10.1073/pnas.85.18.6816
- Tang CK, Draper DE. Unusual mRNA pseudoknot structure is recognized by a protein translational repressor. Cell 1989; 57:531-6; PMID:2470510; http://dx.doi.org/10.1016/0092-8674(89)90123-2
- Brierley I, Boursnell ME, Binns MM, Bilimoria B, Blok VC, Brown TD, Inglis SC. An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J 1987; 6:3779-85; PMID:3428275
- Baranov PV, Gurvich OL, Fayet O, Prere MF, Miller WA, Gesteland RF, Atkins JF, Giddings MC. RECODE: a database of frameshifting, bypassing and codon redefinition utilized for gene expression. Nucleic Acids Res 2001; 29:264-7; PMID:11125107; http://dx.doi.org/10.1093/nar/29.1.264
- Bekaert M, Firth AEA, Zhang Y, Gladyshev VN, Atkins JF, Baranov PV. Recode-2: new design, new search tools, and many more genes. Nucleic Acids Res 2010; 38:D69-74; PMID:19783826; http://dx.doi.org/10.1093/nar/gkp788
- Girnary R, King L, Robinson L, Elston R, Brierley I. Structure-function analysis of the ribosomal frameshifting signal of two human immunodeficiency virus type 1 isolates with increased resistance to viral protease inhibitors. J Gen Virol 2007; 88:226-35; PMID:17170455; http://dx.doi.org/10.1099/vir.0.82064-0
- Kollmus H, Hentze MW, Hauser H. Regulated ribosomal frameshifting by an RNA-protein interaction. RNA 1996; 2:316-23; PMID:8634912
- Yu CH, Noteborn MH, Pleij CW, Olsthoorn RC. Stem-loop structures can effectively substitute for an RNA pseudoknot in -1 ribosomal frameshifting. Nucleic Acids Res 2011; 39:8952-9; PMID:21803791; http://dx.doi.org/10.1093/nar/gkr579
- Su L, Chen L, Egli M, Berger JM, Rich A. Minor groove RNA triplex in the crystal structure of a ribosomal frameshifting viral pseudoknot. Nat Struct Biol 1999; 6:285-92; PMID:10074948; http://dx.doi.org/10.1038/6722
- Clark MB, Janicke M, Gottesbuhren U, Kleffmann T, Legge M, Poole ES, Tate WP. Mammalian gene PEG10 expresses two reading frames by high efficiency -1 frameshifting in embryonic-associated tissues. J Biol Chem 2007; 282:37359-69; PMID:17942406; http://dx.doi.org/10.1074/jbc.M705676200
- Ono R, Kobayashi S, Wagatsuma H, Aisaka K, Kohda T, Kaneko-Ishino T, Ishino F. A retrotransposon-derived gene, PEG10, is a novel imprinted gene located on human chromosome 7q21. Genomics 2001; 73:232-7; PMID:11318613; http://dx.doi.org/10.1006/geno.2001.6494
- Manktelow E, Shigemoto K, Brierley I. Characterization of the frameshift signal of Edr, a mammalian example of programmed -1 ribosomal frameshifting. Nucleic Acids Res 2005; 33:1553-63; PMID:15767280; http://dx.doi.org/10.1093/nar/gki299
- Dinman JD, Wickner RRB. Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered -1 ribosomal frameshifting efficiencies. Genetics 1994; 136:75-86; PMID:8138178
- Baranov P V, Gesteland RF, Atkins JF. Recoding: translational bifurcations in gene expression. Gene 2002; 286:187-201; PMID:11943474; http://dx.doi.org/10.1016/S0378-1119(02)00423-7
- Michel AM, Choudhury KR, Firth AE, Ingolia NT, Atkins JF, Baranov PV. Observation of dually decoded regions of the human genome using ribosome profiling data. Genome Res 2012; 22:2219-29; PMID:22593554; http://dx.doi.org/10.1101/gr.133249.111
- Lyngsø RB, Pedersen CNS. Pseudoknots in RNA secondary structures. In Proc Fourth Annu Int Conf Comput Mol Biol 2000; 201-9.
- Hammell AB, Taylor RLC, Peltz SW, Dinman JD. Identification of putative programmed -1 ribosomal frameshift signals in large DNA databases. Genome Res 1999; 9:417-27; PMID:10330121
- Jacobs JL, Belew AT, Rakauskaite R, Dinman JD. Identification of functional, endogenous programmed -1 ribosomal frameshift signals in the genome of Saccharomyces cerevisiae. Nucleic Acids Res 2007; 35:165-74; PMID:17158156; http://dx.doi.org/10.1093/nar/gkl1033
- Belew AT, Hepler NL, Jacobs JL, Dinman JD. PRFdb: a database of computationally predicted eukaryotic programmed -1 ribosomal frameshift signals. BMC Genomics 2008; 9:339; PMID:18637175; http://dx.doi.org/10.1186/1471-2164-9-339
- Plant EP, Wang P, Jacobs JL, Dinman JD. A programmed -1 ribosomal frameshift signal can function as a cis-acting mRNA destabilizing element. Nucleic Acids Res 2004; 32:784-90; PMID:14762205; http://dx.doi.org/10.1093/nar/gkh256
- Belew AT, Advani VM, Dinman JD. Endogenous ribosomal frameshift signals operate as mRNA destabilizing elements through at least two molecular pathways in yeast. Nucleic Acids Res 2010; 39:2799-808; PMID:21109528; http://dx.doi.org/10.1093/nar/gkq1220
- Belew AT, Meskauskas A, Musalgaonkar S, Advani VM, Sulima SO, Kasprzak, WK, Shapiro, BA, Dinman JD. Ribosomal frameshifting in the CCR5 mRNA is regulated by miRNAs and the NMD pathway. Nature 2014; 512:265-9; PMID:25043019; http://dx.doi.org/10.1038/nature13429
- Dinman JD, Wickner RB. Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered -1 ribosomal frameshifting efficiencies. Genetics 1994; 136:75-86; PMID:8138178
- Dinman JD, O’Connor M. Mutants that affect recoding. In: Atkins JF, Gesteland RF, editors. Recoding: Expansion of Decoding Rules Enriches Gene Expression. New York, Dordrecht, Heidelberg, London.: Springer; 2010. page 321-44.
- Lundblad V, Morris DK. Programmed translational frameshifting in a gene required for yeast telomere replication. Curr Biol 1997; 7:969-76; PMID:9382847; http://dx.doi.org/10.1016/S0960-9822(06)00416-7
- Dahlseid JN, Lew-Smith J, Lelivelt MJ, Enomoto S, Ford A, Desruisseaux M, McClellan M, Lue N, Culbertson MR, Berman J. mRNAs encoding telomerase components and regulators are controlled by UPF genes in Saccharomyces cerevisiae. EukaryotCell 2003; 2:134-42; PMID:12582130
- Enomoto S, Glowczewski L, Lew-Smith J, Berman JG. Telomere cap components influence the rate of senescence in telomerase-deficient yeast cells. Mol Cell Biol 2004; 24:837-45; PMID:14701754; http://dx.doi.org/10.1128/MCB.24.2.837-845.2004
- Lew JE, Enomoto S, Berman J. Telomere length regulation and telomeric chromatin require the nonsense- mediated mRNA decay pathway. Mo lCell Biol 1998; 18:6121-30; PMID:9742129
- Teo SH, Jackson SP. Telomerase subunit overexpression suppresses telomere-specific checkpoint activation in the yeast yku80 mutant. EMBO Rep 2001; 2:197-202; PMID:11266360; http://dx.doi.org/10.1093/embo-reports/kve038
- Mozdy AD, Cech TR. Low abundance of telomerase in yeast: implications for telomerase haploinsufficiency. RNA 2006; 12:1721-37; PMID:16894218; http://dx.doi.org/10.1261/rna.134706
- Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J 2006; 25:565-74; PMID:16424902; http://dx.doi.org/10.1038/sj.emboj.7600952
- Shore D, Bianchi A. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J 2009; 28:2309-22; PMID:19629031; http://dx.doi.org/10.1038/emboj.2009.195
- Pfeiffer V, Lingner J. Replication of telomeres and the regulation of telomerase. Cold Spring Harb. Perspect. Biol. 2013; 5:a010405; PMID:23543032 doi: 10.1101/cshperspect.a010405
- Li Y, Treffers EE, Napthine S, Tas A, Zhu L, Sun Z, Bell S, Mark BL, van Veelen PA, van Hemert MJ, et al. Transactivation of programmed ribosomal frameshifting by a viral protein. Proc Natl Acad Sci U S A 2014; 1-10.
- Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol 2013; 6:327-37; PMID:23782086; http://dx.doi.org/10.1586/ehm.13.23
- Jack K, Bellodi C, Landry DM, Niederer RO, Meskauskas A, Musalgaonkar S, Kopmar N, Krasnykh O, Dean AM, Thompson SR, et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell 2011; 44:660-6; PMID:22099312; http://dx.doi.org/10.1016/j.molcel.2011.09.017
- McCann KL, Baserga SJ. Genetics. mysterious ribosomopathies. Science 2013; cited 2014 Jan 21; 341:849-50; PMID:23970686; http://dx.doi.org/10.1126/science.1244156
- Hekman KE, Yu GY, Brown CD, Zhu H, Du X, Gervin K, Undlien DE, Peterson A, Stevanin G, Clark HB, et al. A conserved eEF2 coding variant in SCA26 leads to loss of translational fidelity and increased susceptibility to proteostatic insult. Hum Mol Genet 2012; 21:5472-83; PMID:23001565; http://dx.doi.org/10.1093/hmg/dds392
- Caliskan N, Katunin VI, Belardinelli R, Peske F, Rodnina MV. Programmed -1 frameshifting by kinetic partitioning during impeded translocation. Cell 2014; 157:1619-31; PMID:24949973; http://dx.doi.org/10.1016/j.cell.2014.04.041
- Sulima SO, Patchett S, Advani VM, De Keersmaecker K, Johnson AW, Dinman JD. Bypass of the pre-60S ribosomal quality control as a pathway to oncogenesis. Proc Natl Acad Sci 2014; 111:5640-5; PMID:24706786; http://dx.doi.org/10.1073/pnas.1400247111
