Abstract
Cellular contractility regulates tissue cohesion and morphogenesis. In epithelia, E-cadherin adhesion couples the contractile cortices of neighboring cells together to produce tension at junctions that can be transmitted across the epithelium in a planar fashion. We have recently demonstrated that contractility is also patterned in the apical-lateral axis within epithelial junctions. Our findings highlight the role that cytoskeletal regulation plays in controlling the levels of intra-junctional tension. Of note, dysregulation of this apicolateral pattern of tension can drive oncogenic cell extrusion. In this article, we provide a detailed description of the actomyosin cytoskeleton organization during oncogenic extrusion and discuss the implications of cell extrusion in cancer.
Introduction
Cadherin-based cell-cell junctions are mechanically-active structures. This has been most extensively studied in polarized epithelia,Citation1 where E-cadherin receptors form adhesive clusters that are coupled to the contractile actomyosin cortex.Citation2,3 At the cellular level, cadherin adhesion not only bind cells together but also mechanically couples the contractile cortices of neighboring cells together to yield junctional tension.Citation1 This tension can serve several morphogenetic purposes, being implicated in apical constrictionCitation4 neighbor exchange during gastrulationCitation5 and also propagated across cells to influence tissue-level morphogenesis.Citation6 Adhesive coupling of cortical contractility has also been proposed to underlie the fundamental process of cell sorting, as cells that generate higher junctional tension segregate away from cells with lower cadherin-based tension.Citation1,7 Ultimately, this can reflect the action of mechanisms that either couple cadherins to the contractile apparatus of cells, or the processes that regulate contractility.Citation2 For instance, cells expressing cadherins that are uncoupled from the actin cortex sort out from cells where adhesion is linked to the cortex.Citation7 Thus, the mechanisms that regulate the integration of adhesion and contractility may affect many aspects of tissue organization.Citation2,8
Patterning Junctional Tension in the Apical-Lateral Axis
In many of the aforementioned studies, junctional tension has been treated as a property that only localizes at the apical junctions between cells,Citation5,9 or as a uniform property of cell-cell contacts.Citation7 However, we recently observed that polarized epithelial monolayers can generate more complex patterns of contractile tension that ultimately influence cellular integration into the epithelium.Citation1,10 This finding derived from the initial observation that E-cadherin clusters are found throughout junctions between cells.Citation1,2,11 At the apical-lateral interface, clusters of E-cadherin are stabilized and concentrate in a ring-like structure that we and others have described as a zonula adherens.Citation12–14 However, extensive E-cadherin was also found distributed in clusters throughout the lateral adherens junctions (LAJ) located below the ZA.Citation15,16 In both cases, E-cadherin clusters were mechanically coupled to a contractile actomyosin apparatus. At the ZA, actomyosin organized to form prominent apical bundles that run contiguous to the ZA,Citation17 whereas actomyosin formed a less aligned network at the LAJ.Citation16,18,19 Both actomyosin networks required junctional E-cadherin and appear to derive from a combination of cadherin-based actin assemblyCitation20,21 and Rho-ROCK signaling.Citation22-24 However, contractile tension was consistently greater at the ZA than at the LAJ. Thus, cells were able to generate distinct zones of tension in the apical-lateral axis of their junctions.Citation1
We are coming to realize that many molecular processes collaborate to determine junctional tension. These include the mechanisms responsible for building actin networks at junctions and the signaling pathways that regulate myosin II. For example, apical tension at the ZA was compromised by inhibiting myosin II or its upstream regulators (ROCK, Rho and Ect2, the activator of Rho at the ZA)Citation25 and also perturbed when the Arp2/3 actin nucleator was blocked.Citation26a We found that actin filament stability was a key to understanding how cells generate different zones of tension at the ZA and LAJ.Citation1 F-actin stability at the LAJ was significantly lower than that found at the ZA, being distinguished by continuous cycles of formation and disassembly at different areas of the lateral cadherin contact.Citation16 This was due to stress-induced actin filament turnover.Citation1,Citation26b Effectively, myosin contractility initially condensed actin networks, leading to the generation of contractile tension, but eventually promoted turnover of the actin filaments that are needed to generate force.Citation16 This represents an intrinsic mechanism for contractile stresses at the LAJ to be readily dissipated.
A further implication was that mechanisms might exist at the apical ZA to stabilize filaments and resist stress-induced filament turnover.Citation1,Citation26b We have identified that this mechanism involves the actin regulator N-WASP. N-WASP is multidomain protein that mediates signal regulation of the actin cytoskeleton.Citation27 It is best understood for its ability to activate actin nucleation by the Arp2/3 complex. Strikingly, N-WASP localizes selectively to the ZA rather than the LAJ and regulates the actin cytoskeleton at the ZA. However, it achieves this by a mechanism that is distinct from Arp2/3 activation.Citation28 Instead, N-WASP stabilizes nascent actin filaments at the ZA, through a molecular pathway that involves the WIP family protein, WIRE.Citation28 Its contribution to junctional contractility was demonstrated by the observation that apical junctional tension is reduced to the levels seen at the LAJ when N-WASP was depleted by RNAi.Citation1 Further, ectopic targeting of active N-WASP to the LAJ increased tension at that site. Thus actin stabilization represents an independent mechanism to modulate contractile stress at cadherin junctions. How it cooperates with the other determinants of junctional contractilityCitation21,29 will be an interesting question for future research.
Junctional Contractility in Cell Extrusion
Precise control over the levels of contractile tension at cadherin junctions is critical in a variety of morphogenetic process including cell-sorting,Citation7 Indeed, we have demonstrated that increasing the cadherin based tension of a cell by stabilizing F-actin throughout the junctional cortex, can lead to its expulsion from its original epithelial population that has reduced levels of cadherin-based tension.Citation1 Based on these observations, we then asked how patterns of intra-junctional tension might contribute to epithelial homeostasis. We focused on the process of apical cell extrusion. Cell extrusion is a fascinating process where minorities of cells become actively expelled from epithelia. First described during apoptosis,Citation30 it has been documented in an increasingly broad range of contexts, including bacterial infection,Citation31 epithelial overcrowdingCitation32 and cellular transformation.Citation33 The latter example illustrates some of the salient features of the extrusion process. Firstly, it only occurs when a single transformed cell is surrounded by non-transformed cells. Secondly, extrusion is a mechanically active process.Citation1 Contractility is necessary for oncogenic extrusion to occur, as is the case for other forms of extrusion.Citation2,30 The requirement for contractility is often thought to be located in the neighboring cells, which exert forces that drive extrusion,Citation32,34,35 Nonetheless, some feature(s) of the transformed cell appears to be recognized by the surrounding cells to elicit a response from the neighbors.Citation36,37 Thus oncogenic extrusion involves complex interplay between transformed cells and their surrounding, non-transformed neighbors.
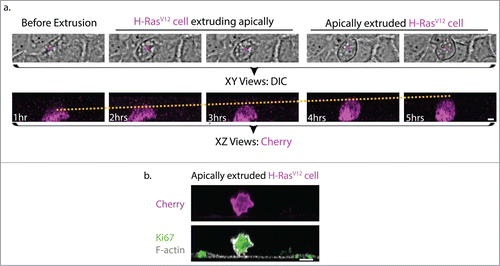
Oncogenic extrusion can be readily elicited when single Caco-2 cells are engineered to express H-RasV12 within a confluent monolayer; in contrast H-RasV 12 expression in more than 3 cells tends to compromise epithelial morphology but did not cause extrusion (data not shown). These single transfected cells exhibited a gradual increase in cell height until they were eventually extruded apically (). Once extruded, non-transformed cells commonly die through anoikisCitation38 when they are extruded, but transformed cells can continue to proliferate,Citation37 as detected by markers such as Ki67 stainingCitation33,39 (). Ras mutations have been shown to allow metastatic tumor cells to evade anoikis by up-regulation of survival signalsCitation40 that can allow cells to proliferate even in the absence of extracellular matrix attachment.Citation41
Figure 1. Morphological comparisons between H-RasV12 expressing cell undergoing extrusion and H-RasV12 expressing cell that has been extruded. (a) Still images of brightfield (top panel, magenta asterisk indicates H-RasV12 expressing cell) and confocal fluorescence XZ cross-sections (bottom panel) from live imaging of single H-RasV12 expressing Caco-2 cells within a wild-type epithelia monolayer. The yellow dotted line in the fluorescence XZ cross-sections indicates the gradual increase in the height of the single H-RasV12 expressing cell as extrusion takes place. Based on the relative height of the extruding cell to its neighboring cell, the process of extrusion can be classified into 3 stages: Before extrusion, H-RasV12 cell extruding apically and apically extruded H-RasV12 expressing cell. (b) Immunofluorescence XZ cross-section of an apically extruded H-RasV12 expressing cell that is loosely attached on the untransfected epithelia monolayer and stained for the proliferation marker Ki67. Scale Bar: 5 μm.
Morphologically, it is possible to distinguish 2 broad phases to this oncogenic extrusion: an extrusion phase where the cell remains largely within the monolayer, and a post-extrusion phase where the cell has escaped, but remains loosely attached to the monolayer (). We focused on analyzing the extrusion phase, as this seemed most likely to reveal the molecular mechanisms and cortical mechanics that drive cell extrusion.Citation1 This stage is associated with distinct changes in both E-cadherin and F-actin distribution (). The apical E-cadherin ring representing the ZA became disrupted, as other have also observed.Citation36,42 However, clusters of homoligated cadherin persisted throughout the contact zones between the cells, including the LAJCitation1 (), suggesting that adhesion was preserved until later in the extrusion process. Similarly, the prominent bundles of apical F-actin were lost (). Instead, extruding cells display an intense cortical F-actin network throughout their contact zones. This suggested that regulation of F-actin was being altered within the oncogene-expressing cell itself. Indeed, we observed that N-WASP was redistributed at the contact zone, being depleted from the apical region (which may contribute to loss of the ZACitation28 and increasing at the lateral zone (). This is accompanied by a redistribution of WIRE (), which mediates F-actin stabilization by N-WASP.Citation28
Figure 2. Epithelial organization during the stages of oncogenic cell extrusion. (a) Immunofluorescence staining of E-cadherin (green) and DAPI (blue). XZ cross-section of an H-RasV12 expressing cell extruding apically (left panel), and an H-RasV12 expressing cell that has extruded and is loosely attached to the monolayer (right panel). Red dotted line indicates the location of the Z plane for the extruded region, cyan dotted line indicates the apical Z plane of neighboring cells and yellow dotted line indicates the basolateral Z plane of the Caco-2 monolayer. (b) Confocal XY sections of (a) from an H-RasV12 expressing cell extruding apically (left panel) and an extruded H-RasV12 expressing cell that remains loosely attached to the monolayer (right panel). Magenta asterisk indicates the position of the H-RasV12 expressing cell. (b’) Magnified image of the basolateral cell-cell contacts between the extruding cells (H-RasV12, Magenta) and neighboring cells as indicated by a white box in (b). Immunofluorescence staining of E-cadherin (green), F-actin (phalloidin, red), DAPI (blue) and the merged image of E-cad and F-actin. Scale Bars: 5 μm.
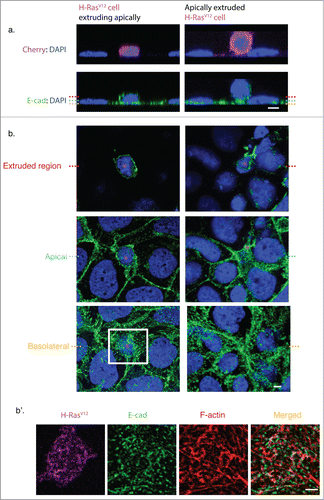
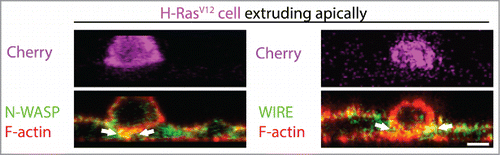
Figure 3. N-WASP-WIRE mediated lateral F-actin stabilization during the single outgrowth of H-RasV12 expressing cell. Immunofluorescence XZ cross-section of a H-RasV12 –Cherry (magenta) expressing cell that is in the process of extrusion also stained for F-actin (phalloidin, red), N-WASP (green) or WIRE (green). The white arrows in the image indicate the concentration of colocalized N-WASP/WIRE with F-actin at the basolateral interface of wild type and transformed cell during apical extrusion. Scale Bars: 5 μm.
This suggested that the redistribution of N-WASP might be accompanied by altered junctional tension. Indeed, we found that the junctional interface between H-RasV12-transfected and untransfected cells displayed both decreased tension in the apical region and increased tension in the lateral zone.Citation1 This observation was in agreement with the previous finding that N-WASP can modulate junctional tension by stabilizing F-actin. Further, the increased cortical F-actin seen in cells undergoing extrusion is accompanied by an increase in activated Myosin II, consistent with redistribution of a contractile cortex (). Indeed when H-RasV12-transfected cells were also depleted of N-WASP, the lateral accumulation of F-actin and active Myosin II was abolished, lateral junction tension did not increase, and oncogenic extrusion was inhibited (). This implied that N-WASP's impact on the junctional actomyosin network was necessary for the extrusion process to occur. Further, we found that extrusion could be elicited by manipulating the distribution of N-WASP to mimic the altered patterns of junctional tension seen with oncogene transfection.Citation1 Overall, these results revealed that altering regional actin dynamics within cell-cell junctions regulated cortical contractility within oncogene-transfected cells to drive cell extrusion ().
Figure 4. N-WASP supports F-actin and Myosin II accumulation at the lateral contact between wild type and a H-RasV12 expressing cells to drive oncogenic extrusion. (a) Immunofluorescence staining of phosphoMyosin light chain (green), F-actin (phalloidin, red), DAPI (blue) and the merged of phosphoMyosin regulatory light chain (green) and F-actin (phalloidin, red). Representative XZ cross-section of a H-RasV12 expressing cell extruding apically (left panel of scramble control), an extruded H-RasV12 expressing cell that is loosely attached to the wild type monolayer (right panel of scramble control); and N-WASP knockdown monolayer with a H-RasV12 expressing cell surrounded by wild type cells. (b) Comparison of Myosin regulatory light chain phosphorylation intensity at the lateral junctions between H-RasV12 expressing and neighbor cells in control and N-WASP knockdown monolayers. (c) Comparison of F-actin intensity at the lateral junctions between H-RasV12 expressing and neighbor cells in control and N-WASP knockdown monolayers.
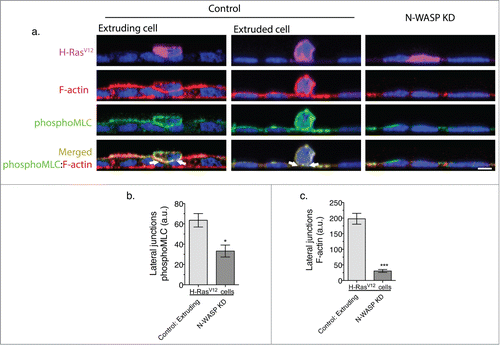
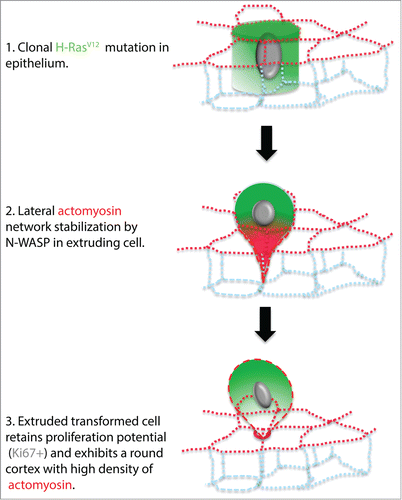
Figure 5. Model depicting the apical extrusion of a H-RasV12 expressing cell from the epithelium. (1) H-RasV12 clonal mutation in a single cell within the epithelium. The H-RasV12 expressing cell initially remains in the epithelium. (2) Subsequently, lateral F-actin stabilization by N-WASP promotes the recruitment of active myosin that leads to the constriction of the basolateral portion of H-RasV12 expressing cell. (3) The H-RasV12 mutant cell is then fully extruded apically by the concentration of actomyosin at the base of the extruded cell, which is located at the apical plane of the surrounding epithelia cells. The apically extruded cell remains loosely attached to the wild type monolayer and can proliferate.
Implications for Oncogenic Cell Extrusion
Our findings have several interesting implications for future research. Firstly, the mechanical processes that drive cell extrusion can involve the extruding cells as well as their neighbors. Much current work, deriving especially from the analysis of apoptotic extrusion, has focused attention on how contractility in neighboring cells may induce extrusion. Prominent contractile purse strings have been identified in the neighboring cells that are thought to drive extrusion,Citation32,43 under regulation by dynamic microtubules and their control of cortical Rho signaling.Citation34 Our findings complement other recent reports that suggest an active role for the cellular cortex of the extruding cell. For example, cortical F-actin content is increased specifically in the extruding transformed cell rather than in the surrounding cells,Citation42 changes which may contribute to the observation by atomic force microscopy that the H-RasV12 -transformed cells are stiffer than the neighboring untransfected cells.Citation37 Contractile pulsations of junctions were also altered in Drosophila amnioserosa cells that underwent naturally-occurring delamination.Citation44 Additionally, work on UV-induced apoptosis has also delineated a mechanical role for the extruding cells to initiate extrusion.Citation43 Here, the actin cytoskeleton at the ZA of the apoptotic cell pulls on its neighbors, to trigger the upregulation of lateral F-actin in those neighbors which expels the apoptotic cell.Citation43 Broadly, this implies that we need to consider how mechanical changes in the extruding cell may actively cooperate with changes in the neighboring cells to account for the extrusion process. Also, to an extent it has been tempting to treat all forms of extrusion as equivalent phenomena; however, it remains possible that different mechanisms may contribute to different forms of extrusion. For instance, one distinguishing feature to consider is that permeabilization of the cellular membrane occurs in apoptotic extrusionCitation43 but is not evident in oncogenic extrusion; this difference may lead to different contractile responses in these 2 circumstances.
Secondly, oncogenic extrusion has interesting implications for cancer biology. Of note, oncogenic extrusion not only occurs in transformed cells within normal tissuesCitation45,46 but also transformed cells that acquire additional oncogenic mutations.Citation1,47 Furthermore, it seems reasonable to postulate that many stages in the life cycle of an epithelial tumor will involve an interaction between minority and majority populations of cells, where those minorities will bear new mutations not shared with the majority. Indeed, the initiation of carcinogenesis has been thought to arise from oncogenic mutations of a single cell within tissuesCitation33,48,49 and it is predicted that successive genetic changes will first occur in a minority of cells before they achieve a growth advantage.Citation50 This raises the question of whether the extrusion process, which occurs when normal cells surround transformed cell, can contribute to tumorigenesis. Indeed, there is emerging evidence to suggest that this may be the case. Leung and BruggeCitation33 demonstrated that single-oncogene expressing mammary epithelial cells did not proliferate until they had been extruded from the normal breast epithelia. This could reflect contact inhibition of proliferation by E-cadherin adhesion. Moreover when extrusion did not occur as the entire acini expressed the oncogene, these transformed cells remained in their growth-arrested phase. Thus, although extrusion has often been portrayed as a mechanism for tissues to clear cells from an epitheliumCitation35,31,43(Marinari et al., 2012), it is possible that it is coopted by transformed cells to facilitate tumorigenesis.
Although it often occurs in an apical direction, oncogenic extrusion in vitro can occur basally,Citation46,51 which, in a tissue context, would be directed toward the body compartment. This raises the interesting issue of whether the mechanical process of extrusion might contribute to early phases of tumor invasion. Morphological features resembling extrusion are documented in early tumors,Citation33,51,52 but remain to be thoroughly analyzed. If extrusion were to contribute to tumor invasion in mammals, the direction of extrusion must then be regulated. Indeed, Marshall et al 2011Citation47 reported that apoptotic cells could be induced to change their direction of extrusion, from apical to basal, by depletion of the Adenomatosis Polyposis Coli (APC) gene product, which is a major target in the genetic progression of colon cancer.Citation53 Whether oncogenes contribute to tumor progression by regulating the morphogenetic process of extrusion, as well as perturbing proliferative control, is an interesting hypothesis for the future.
Finally, the role of cortical mechanics in oncogenic extrusion raises the interesting question of whether effective therapeutics can be developed that target cellular processes coopted by oncogene products. Experimental studies have identified roles for N-WASP in the regulation of tumor cell migration and invasion.Citation54,55 However, N-WASP is not commonly identified in screens for putative tumor-inducing genes with coding mutations or that are misexpressed. One potential reason is that actin-regulatory genes, such as N-WASP, may be dysregulated and functionally coopted by oncogenic signaling, but not direct genetic targets during tumorigenesis. Nonetheless, they may be necessary for tumor cell extrusion, migration and invasion.Citation54,55 If so, then maneuvers that target their function may provide the opportunity to develop therapeutics that are orthogonal to those which are directed against their upstream oncogene products. Of course, these are interesting speculations for the future. Nonetheless, they highlight the capacity for oncogenic extrusion, and its regulation by active junctional mechanics, to contribute to a better understanding of tumor cell biology.
Materials and Methods
Cell culture and transfection
Caco-2 cells were procured from ATCC (HTB-37). Caco-2 cultures were routinely cultured in RPMI supplemented with 10% FBS, 1% non-essential amino acids, 1% L-glutamine, 1% w/v Penicillin/Streptomycin and low doses of plasmocin (Invivogen). Plasmid DNA transfections were performed at 40–60% cell confluency using Lipofectamine 3000 (Invitrogen) according to the manufacturers’ instructions and analyzed 24–48 hours post transfection (at 100% confluency). Live-cell imaging was performed on cells grown on 29 mm glass-bottomed dishes (Shengyou Biotechnology Co. Ltd, China) and the RPMI was replaced with clear Hanks Balanced Salt Solution supplemented with 5% FBS, 10 mM HEPES pH 7.4 and 5 mM CaCl2, during imaging.
To evaluate the frequency of oncogenic apical extrusion, cells were co-transfected at 80% confluency with 0.2μg/ml of mCherry-H-RasV12 and with either scrambled siRNA or N-WASP siRNA. Single cells co-expressing H-RasV12 with other transgenes surrounded by H-RasV12 null expressing cells were then analyzed at 36 hours post transfection. Only cells with an intact nucleus were quantitated.
Antibodies and immunofluorescence
Cells were fixed at 4°C with parformaldehyde on ice for 5 min. Primary antibodies in this study were: mouse anti-human ectodomain E-cadherin antibody (1:50; clone# NCC-CAD-299, a gift from P.Wheelock, University of Nebraska, Omaha, USA, with the permission of M.Takeichi); rabbit monoclonal antibody 30D10 against N-WASP (1:50; Cell Signaling Technologies; cat#30D10); rabbit polyclonal antibody against GAPDH (1 in 4000; Trevigen; cat#2275-PC-100); rabbit polyclonal antibody against WIRE (1:50; HPA024467; Sigma Aldrich); mouse monoclonal antibody against Serine 19 of Phospho Myosin Light Chain 2 (1:100; Cell Signaling Technologies; cat#3675). Alexa Fluor conjugated secondary antibodies were from Invitrogen.
Confocal images were captured with a Zeiss 710 laser-scanning confocal microscope. Time-lapse images of brightfield and mCherry fluorescence were acquired using a ×40 objective, 1.3 NA oil Plan-Apochromat immersion lens and 6 Z-stacks of 1μm step size.
Image processing and analysis
The images presented were processed with ImageJ (http://rsb.info.nih.gov/ij/) and Photoshop CS (Adobe Systems, Inc.). Image size was then increased to smoothen movies upon conversion into H.264 compression format. The edges of XZ images were increased using ImageJ canvas size function to uniformly align images for representation purposes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all our lab colleagues for their support and advice during this work, and our colleagues elsewhere for the generous gifts of reagents. Confocal and optical microscopy was performed at the ACRF Cancer Biology Imaging Facility, established with the generous support of the Australian Cancer Research Foundation.
Funding
This work was supported by the National Health and Medical Research Council of Australia through grants and research fellowships to AY (631383, 1010489, 1037320, 1044041, 1067405), the Australian Research Council (DP120104667), the EMPathy National Collaborative Research Program (CG-10–04) of the National Breast Cancer Foundation (Australia), The Kids Cancer Project of The Oncology Children's Foundation, The University of Queensland Early Career Grant (2012003354) to GAG and a University of Queensland Postdoctoral Fellowship to AKL
References
- Wu SK, Gomez GA, Michael M, Verma S, Cox HL, Lefevre JG, Parton RG, Hamilton NA, Neufeld Z, Yap AS. Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol 2014; 16:167-78; PMID:4413434
- Wu SK, Yap AS. Patterns in space: coordinating adhesion and actomyosin contractility at E-cadherin junctions. Cell Commun Adhes 2013; 20:201-12; PMID:24205985; http://dx.doi.org/10.3109/15419061.2013.856889
- Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat Cell Biol 2014; 16:587-94; PMID:24859003; http://dx.doi.org/10.1038/ncb2973
- Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, Gao L, Betzig E, Kiehart DP, Goldstein B. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 2012; 335:1232-5; PMID:22323741; http://dx.doi.org/10.1126/science.1217869
- Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 2009; 17:736-43; PMID:19879198; http://dx.doi.org/10.1016/j.devcel.2009.09.003
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J Cell Biol 2010; 188:735-49; PMID:20194639; http://dx.doi.org/10.1083/jcb.200910099
- Maitre JL, Berthoumieux H, Krens SF, Salbreux G, Julicher F, Paluch E, Heisenberg CP. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 2012; 338:253-6; PMID:22923438; http://dx.doi.org/10.1126/science.1225399
- de Rooij J. Cadherin adhesion controlled by cortical actin dynamics. Nat Cell Biol 2014; 16:508-10; PMID:24875740
- Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting drosophila tissue morphogenesis. Nat Cell Biol 2008; PMID:18978783; http://dx.doi.org/10.1038/ncb1798
- Smutny M, Wu SK, Gomez GA, Mangold S, Yap AS, Hamilton NA. Multicomponent analysis of junctional movements regulated by myosin II isoforms at the epithelial zonula adherens. PloS one 2011; 6:e22458; PMID:21799860; http://dx.doi.org/10.1371/journal.pone.0022458
- Lugo-Martinez VH, Petit CS, Fouquet S, Le Beyec J, Chambaz J, Pincon-Raymond M, Cardot P, Thenet S. Epidermal growth factor receptor is involved in enterocyte anoikis through the dismantling of E-cadherin-mediated junctions. Am J Physiol Gastrointest Liver Physiol 2009; 296:G235-44; PMID:19056766; http://dx.doi.org/10.1152/ajpgi.90313.2008
- Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 2008; 135:948-59; PMID:19041755; http://dx.doi.org/10.1016/j.cell.2008.09.040
- Priya R, Yap AS, Gomez GA. E-cadherin supports steady-state rho signaling at the epithelial zonula adherens. Differentiation 2013; 86:117-8; PMID:23643492; http://dx.doi.org/10.1016/j.diff.2013.01.002
- Truong Quang BA, Mani M, Markova O, Lecuit T, Lenne PF. Principles of E-Cadherin supramolecular organization in vivo. Curr Biol 2013; 23:2197-207; PMID:24184100; http://dx.doi.org/10.1016/j.cub.2013.09.015
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Developmental cell 2002; 3:367-81; PMID:12361600
- Wu SK, Budnar S, Yap AS, Gomez GA. Pulsatile contractility of actomyosin networks organizes the cellular cortex at lateral cadherin junctions. Eur J Cell Biol 2014; 93:396-404; PMID:25269995; http://dx.doi.org/10.1016/j.ejcb.2014.09.001
- Mangold S, Wu SK, Norwood SJ, Collins BM, Hamilton NA, Thorn P, Yap AS. Hepatocyte growth factor acutely perturbs actin filament anchorage at the epithelial zonula adherens. Curr Biol 2011; 21:503-7; PMID:21396819; http://dx.doi.org/10.1016/j.cub.2011.02.018
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol 2006; 175:135-46; PMID:17015620
- Tang VW, Brieher WM. Alpha-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol 2012; 196:115-30; PMID:2232703
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 2002; 12:379-82; PMID:11882288
- Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr biol 2014; 24:1689-99; PMID:25065757; http://dx.doi.org/10.1016/j.cub.2014.06.028
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 2005; 16:4531-42; PMID:16030252
- Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res 2004; 295:300-14; PMID:15093731
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 2010; 12:696-702; PMID:20543839; http://dx.doi.org/10.1038/ncb2072
- Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and α-catenin regulate rho signalling at the epithelial zonula adherens. Nat Cell Biol 2012; 14:818-28; PMID:22750944; http://dx.doi.org/10.1038/ncb2532
- (a) Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell 2012; 23:4601-10; PMID:23051739; http://dx.doi.org/10.1091/mbc.E12-08-0574; (b) Moore T, Wu SK, Michael M, Yap AS, Gomez GA, Neufeld Z. Self-organizing actomyosin patterns on the cell cortex at epithelial cell-cell junctions. Biophysical journal 2014; 107:2652-61.
- Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Ann Rev Biochem 2010; 79:707-35; PMID:20533885; http://dx.doi.org/10.1146/annurev.biochem.77.060407
- Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat Cell Biol 2011; 13:934-43; PMID:21785420; http://dx.doi.org/10.1038/ncb2290
- Han SP, Gambin Y, Gomez GA, Verma S, Giles N, Michael M, Wu SK, Guo Z, Johnston W, Sierecki E, et al. Cortactin scaffolds Arp2/3 and WAVE2 at the epithelial zonula adherens. J Biol Chem 2014; 289:7764-75; PMID:24469447; http://dx.doi.org/10.1074/jbc.M113.544478
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 2001; 11:1847-57; PMID:11728307
- Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. Dissemination of invasive salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Nat Acad Sci U S A 2010:17733-8; PMID:20876119; http://dx.doi.org/10.1073/pnas.1006098107
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 2012; 484:542-5; PMID:22504180; http://dx.doi.org/10.1038/nature10984
- Leung CT, Brugge JS. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 2012; 482:410-3; PMID:22318515; http://dx.doi.org/10.1038/nature10826
- Slattum G, McGee KM, Rosenblatt J. P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J Cell Biol 2009; 186:693-702; PMID:19720875; http://dx.doi.org/10.1083/jcb.200903079
- Kajita M, Sugimura K, Ohoka A, Burden J, Suganuma H, Ikegawa M, Shimada T, Kitamura T, Shindoh M, Ishikawa S, et al. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat Commun 2014; 5:4428; PMID:25079702; http://dx.doi.org/10.1038/ncomms5428
- Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, Charras G, Tada M, Fujita Y. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci 2010; 123:171-80; PMID:20026643; http://dx.doi.org/10.1242/jcs.057976
- Hogan C, Dupre-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-Lopez LA, Vincent JP, Itoh Y, et al. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol 2009; 11:460-7; PMID:19287376; http://dx.doi.org/10.1038/ncb1853
- Liotta LA, Kohn E. Anoikis: cancer and the homeless cell. Nature 2004; 430:973-4; PMID:15329701
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182:311-22; PMID:10653597
- McFall A, Ulku A, Lambert QT, Kusa A, Rogers-Graham K, Der CJ. Oncogenic ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol Cell Biol 2001; 21:5488-99; PMID:11463831
- Thullberg M, Gad A, Le Guyader S, Stromblad S. Oncogenic H-Ras V12 promotes anchorage-independent cytokinesis in human fibroblasts. Proc Nat Acad Sci U S A 2007; http://dx.doi.org/104:20338-43; PMID:18077377
- Grieve AG, Rabouille C. Extracellular cleavage of E-cadherin promotes epithelial cell extrusion. J Cell Sci 2014; 127:3331-46; PMID:24895403; http://dx.doi.org/10.1242/jcs.147926
- Kuipers D, Mehonic A, Kajita M, Peter L, Fujita Y, Duke T, Charras G, Gale JE. Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J Cell Sci 2014; 127:1229-41; PMID:24463819; http://dx.doi.org/10.1242/jcs.138289
- Saravanan S, Meghana C, Narasimha M. Local, cell-nonautonomous feedback regulation of myosin dynamics patterns transitions in cell behavior: a role for tension and geometry? Mol Biol Cell 2013; 24:2350-61; PMID:23741052; http://dx.doi.org/10.1091/mbc.E12-12-0868
- Hogan C, Kajita M, Lawrenson K, Fujita Y. Interactions between normal and transformed epithelial cells: their contributions to tumourigenesis. Int J Biochem Cell Biol 2011; 43:496-503; PMID:21187160; http://dx.doi.org/10.1016/j.biocel.2010.12.019
- Liu JS, Farlow JT, Paulson AK, Labarge MA, Gartner ZJ. Programmed cell-to-cell variability in ras activity triggers emergent behaviors during mammary epithelial morphogenesis. Cell Rep 2012; 2:1461-70; PMID:23041312; http://dx.doi.org/10.1016/j.celrep.2012.08.037
- Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell 2011; 22:3962-70; PMID:21900494; http://dx.doi.org/10.1091/mbc.E11-05-0469
- Nowell PC. The clonal evolution of tumor cell populations. Science 1976; 194:23-8; PMID:959840
- Fialkow PJ. Clonal origin of human tumors. Biochim Biophysica Acta 1976; 458:283-321; PMID:1067873
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230
- Slattum G, Gu Y, Sabbadini R, Rosenblatt J. Autophagy in oncogenic K-Ras promotes basal extrusion of epithelial cells by degrading S1P. Curr Biol 2014; 24:19-28; PMID:24361067; http://dx.doi.org/10.1016/j.cub.2013.11.029
- Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Eng J med 2004; 350:1430-41; PMID:15070793
- Fodde R. The APC gene in colorectal cancer. Eur J Cancer 2002; 38:867-71; PMID:11978510
- Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, Schwarz JP, Spence H, Futo K, Timpson P, et al. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol 2012; 199:527-44; PMID:23091069; http://dx.doi.org/10.1083/jcb.201203025
- Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci 2012; 125:724-34; PMID:22389406; http://dx.doi.org/10.1242/jcs.092726
