Prostaglandins regulate a wide variety of physiological and pathological processes, including inflammation, reproduction, cardiovascular homeostasis, and cancer progression. Cyclooxygenase (COX) catalyzes the rate-limiting step in the production of prostaglandins from arachidonic acid (). In 2 reaction steps, arachidonic acid is firstly converted to PGH2, subsequently metabolized to form structurally related prostanoids in various tissues, including PGE2, PGD2, PGF2α, PGI2 and Thromboxane A2 (TxA2).Citation1 Until now, most of the studies evaluating prostaglandin signaling have come from mammalian models. We propose that zebrafish provides a useful model system to elucidate the roles of prostaglandin signaling in embryogenesis and organ development.
Figure 1. Prostaglandin signaling regulates ciliogenesis by modulating intraflagellar transport. PGE2 is synthesized by COX1 and COX2 and then exported by LKT/ABCC4 transporter. Released PGE2 binds to EP4 receptor on the cilium, which activates adenylate cyclase (AC) to promote accumulation of intracellular cAMP. The cAMP-dependent PKA phosphorylates as-yet unknown substrates, leading to increase anterograde IFT and promote ciliogenesis.
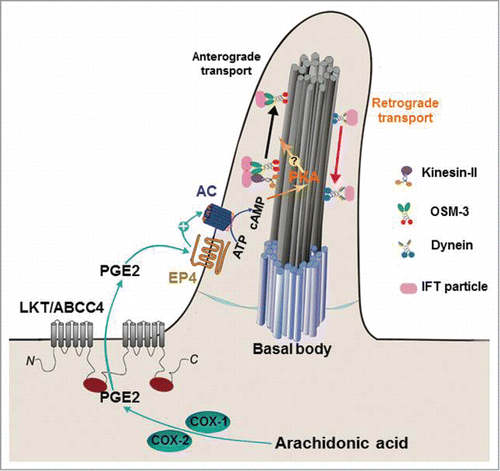
Through analyzing a zebrafish mutant leakytail (lkt), we demonstrate that cilia formation and elongation require prostaglandin signaling during development.Citation2 Our findings indicate that Lkt/ABCC4-mediated PGE2 signaling affects cAMP level and promotes ciliogenesis. A model is proposed where PGE2 is exported from cells via Lkt/ABCC4 transporter on the plasma cell membrane and signals through the G-protein coupled receptor EP4 on the cilium or at the base of the cilium, thereby activating adenylate cyclase (AC) and cAMP signaling to promote the anterograde intraflagellar transport (IFT)2 (). In support of this model, we found that PGE2 treatment causes an increase of intracellular cAMP but not Ca2+ in inner medullary collecting duct 3 (IMCD3) cells during ciliogenesis.Citation2 PGE2 treatment increases anterograde velocities of IFT particles but has no obvious effects on retrograde velocitiesCitation2 (). We further observed that deficiency in Lkt/Abcc4 or Ep4 activities failed to alter expression of the ciliary transcriptional factor Foxj1,Citation2 suggesting that PGE2 signaling regulates ciliogenesis through facilitating the axonemal assembly rather than cilia biosynthesis. These findings linking PGE2 signaling to the second messenger represent a key regulatory step in the control of the anterograde IFT during ciliogenesis.
Cilia formation and elongation require coordinate regulation of bidirectional traffic of IFT particles, including frequency and speed. One plausible mechanism in control of IFT speed during ciliogenesis can be inferred from studies of the nematode cilium, where 2 motors, kinesin-II and OSM3, act in a concerted fashion and produce an intermediate speed in lengthening ciliaCitation3 (). Deletion of the fast motor OSM3 causes IFT particles to move at the slow velocity and ablation of the slow motor Kinesin-II results in IFT particles to move at the fast velocity.Citation3 A mechanism to enzymatically modulates motor or IFT proteins could be used to regulate particle velocity. It is unknown whether the motors can be served as substrates of PKA in response to PGE2 (). During the anterograde axonal transport of vesicle in the leg giant axon, several axoplasmic proteins including kinesin can be phosphorylated by cAMP/PKA activation.Citation4 Alternatively, kinesin or cytoplasmic dynein proteins form complexes with PKA subunits in response to PGE2 during IFT. In melanophores, cAMP/PKA regulates intracellular organelle transport during aggregation and dispersion of pigment granules. Motor proteins dynein and kinesin II form 2 separate complexes with PKA regulatory subunits. Removal of PKA from granules causes disruption of dynein-dependent pigment aggregation, leading to kinesin II-dependent pigment dispension.Citation5 This direct contact model appears to be the efficient way to switch between various kinds of intracellular transport.
The roles of PGE2 signaling in ciliary development were not apparent from previous murine genetic studies. This is likely due to the maternal contribution of prostaglandins in the placenta allowing PGE2 deficient mouse embryos to develop normally. We circumvented the maternal interference by using externally developing zebrafish embryos to reveal the roles of Lkt/ABCC4-mediated PGE2 signaling in regulating ciliogenensis and organ laterality. In agreement with animal model studies, cultured mammalian cells deficient in PGE2 signaling display defective ciliogenesis.Citation2 Zebrafish are an attractive model system to reveal the roles of prostaglandins in embryogenesis, stem cell formation and organ development.Citation1 PGE2 signaling has been identified to regulate morphogenetic movements of convergence and extension and is essential for gastrulation movements in zebrafish embryos.Citation1 Importantly, activation of PGE2 signaling expands haematopoietic stem cells in zebrafish and mouse, thereby providing potential therapy for human patients with depleted haematopoietic stem cells. Recent studies indicate that PGE2 activity has a critical role in the specification and outgrowth of liver and pancreas, and can determine the fate of liver versus pancreas progenitor cells.Citation6 These findings place prostaglandin signaling among the canonical protein signaling pathways in regulating embryo development and organ formation, in addition to its long-standing roles in physiology and pathology.
From an evolution perspective, prostaglandins have been found in all major phyla, and can regulate development of metazoans similar to protein signaling pathways.Citation7 Prostaglandins offer unique ancestral advantages compared to protein signaling pathways. Prostaglandins are synthesized de novo from membrane-released arachidonic acid when cells are activated, and they do not need the energy and storage requirements of proteins. The important properties of prostaglandins are their stereochemical precision in recognition, their potency in the nanomolar range and their evanescent half-life. This enables them to have more quick and accurate response in spatial and temporal manners in the setting of development and physiology. Prostaglandin molecules are truly a conundrum. New insight into their roles in development and disease, and the potential therapeutics based on the novel findings will undoubtedly arise in the near future.
References
- Cha YI, et al. Dev Biol 2006; 289 (two):263-72; PMID:16310177; http://dx.doi.org/10.1016/j.ydbio.2005.10.013
- Jin DQ, et al. Nat Cell Biol 2014; 16 (nine):841-51; PMID:25173977; http://dx.doi.org/10.1038/ncb3029
- Pan XY, et al. J Cell Biol 2006; 174 (seven):1035-45; PMID:17000880; http://dx.doi.org/10.1083/jcb.200606003
- Okada Y, et al. J Neurosci 1995; 15 (four):3053-64; PMID:7536826
- Kashina AS, et al. Curr Biol 2004; 14 (20):1877-81; PMID:15498498, http://dx.doi.org/10.1016/j.cub.2004.10.003
- Nissim S, et al. Dev Cell 2014; 28 (four):423-37; PMID:24530296; http://dx.doi.org/10.1016/j.devcel.2014.01.006
- Yuan DJ, et al. BBA-Mol Cell Biol L 2014; 1841 (nine):1272-84.
