Abstract
Among the different transport systems present in plant cells, Shaker channels constitute the major pathway for K+ in the plasma membrane. Plant Shaker channels are members of the 6 transmembrane-1 pore (6TM-1P) cation channel superfamily as the animal Shaker (Kv) and HCN channels. All these channels are voltage-gated K+ channels: Kv channels are outward-rectifiers, opened at depolarized voltages and HCN channels are inward-rectifiers, opened by membrane hyperpolarization. Among plant Shaker channels, we can find outward-rectifiers, inward-rectifiers and also weak-rectifiers, with weak voltage dependence. Despite the absence of crystal structures of plant Shaker channels, functional analyses coupled to homology modeling, mostly based on Kv and HCN crystals, have permitted the identification of several regions contributing to plant Shaker channel gating. In the present mini-review, we make an update on the voltage-gating mechanism of plant Shaker channels which seem to be comparable to that proposed for HCN channels.
Rapid plant growth and development require large fluxes of K+ to growing tissues. In the plant model organism Arabidopsis thaliana, a variety of K+ channels and transporters, differing in transport affinity, energetic coupling, voltage sensitivity or ionic selectivity, are involved in uptake of K+ from the soil and its allocation in different organs.Citation1-4 Among these K+ transport proteins, the Shaker voltage-gated K+ channels are active at the plasma membrane where they dominate K+ membrane conductance in plant cells. In this mini-review the main structural features of K+ Shaker channels are presented in relation with their animal counterparts, the HCN and Kv channels.
Plant Shaker Channels: Similar Structure, Different Voltage-Gating
Functional Shaker channels are multimeric proteins formed by the assembly of 4 α-subunits arranged around a central ion-conducting pore. All plant Shaker subunits analyzed so far possess the same membrane topology: a short N-terminal cytosolic domain followed by 6 transmembrane segments and a large cytosolic C-terminal part (). Two modules can be distinguished in the transmembrane core: a voltage-sensing module comprising the first 4 transmembrane segments (S1-S4) and a pore-forming module (S5-P-S6).Citation5 In the former, the fourth transmembrane segment (S4), which is enriched in positively charged residues, constitutes the voltage sensor. Between the fifth (S5) and sixth (S6) transmembrane segments of the pore-forming module, a pore loop (P) containing the K+ selectivity filter “TxGYG” (Thr-X-Gly-Tyr-Gly) is located. The cytosolic C-terminal part which begins just after the end of the sixth transmembrane segment (S6), contains the following domains successively: a C-linker (about 80 residues in length), a cyclic-nucleotide binding domain (CNBD), an ankyrin domain (absent in some subunits) and a KHA domain rich in hydrophobic and acidic residues.Citation6-9 To form a functional channel, Shaker α-subunits can be assembled into homomeric channels (assembly of identical Shaker subunit gene products) or heteromeric channels (assembly of different Shaker subunit gene products). Homomeric Shaker channels have been well studied in the model plant Arabidopsis thaliana where 8 out of 9 Shaker α-subunits identified in the Arabidopsis genome have been characterized at the functional level. Four of them form inwardly-rectifying channels (opened by membrane hyperpolarisation; KAT1, KAT2, AKT1 and SPIK), 2 form outwardly-rectifying channels (opened by membrane depolarisation; GORK and SKOR), one forms weakly-rectifying channels (weakly sensitive to voltage, AKT2) and the last one named AtKC1, which seems unable to interact with itself to form functional homomeric channels, can interact with inwardly-rectifying and weakly-rectifying subunits to form functional heteromeric channels.Citation4,8 The formation of heterotetramers with inwardly-rectifying, weakly-rectifying and AtKC1 subunits is common and proven to occur in plants.Citation10-12 In contrast, whereas it has been demonstrated in yeast and Xenopus oocytes that SKOR and GORK subunits can physically interact and assemble into heteromeric Kout channels,Citation13 the presence of these latter channels is controversely discussed in planta because no overlap of expression pattern for SKOR and GORK subunits has been reported.Citation8
Figure 1 (See previous page). Membrane topology of plant Shaker, HCN and Kv channels. (A) Plant Shaker α-subunits exhibit a short N-terminal cytosolic tail, 6 transmembrane segments and a long C-terminal cytosolic tail. The fourth transmembrane segment (S4), which is rich in Arginines, acts as a voltage sensor. Between the fifth (S5) and the sixth (S6) transmembrane segment, there is a loop which participates in the formation of the pore (P) and contains the K+ selectivity filter. The C-terminal cytosolic tail consists of a C-linker, a cyclic-nucleotide binding domain (CNBD), an ankyrine domain and a KHA domain. (B) Animal HCN α-subunits share the same basic subunit structure with plant Shaker α-subunits with the exception of the post-CNBD region which is not conserved. (C) Animal Kv α-subunits display the same topology of plant Shaker and HCN α-subunits at the membrane level but the former notably differs in the cytosolic tails. The N-terminal tail is longer than in the other types of subunits and exhibits the tetramerization domain T1. The C-terminal tail is shorter and does not possess any of the domains described above. (D) Aminoacid sequence alignment of selected plant Shaker and mouse HCN2 α-subunits covering the S4-S5 linker and the beginning of the C-linker (putative A′ helix) regions which have been shown to interact and contribute to voltage-gating in HCN channels.Citation24,27 Plant sequences, previously characterized in heterologous systems, are grouped with respect to the voltage-gating properties of the corresponding channels (Kin: inward-rectifiers, Kweak: weak-rectifiers, Ksilent: electrically silent, Kout: outward-rectifiers). Sequence alignment was carried out with MUSCLE (MUltiple Sequence Comparison by Log-Expectation). Gray backgrounds depict identical residues in more than half of the analyzed sequences. Red background depicts conserved residues in the mouse HCN2 sequence. Black background depicts residues mutated in (E). Boxed sequences comprise residues exchanged in KAT2-AtKC1 chimeras described in Nieves-Cordones et al. 2014.Citation9 Protein alignment includes one sequence from mouse (mHCN2 (NP_032252.1)Citation43), 8 from Arabidopsis (SPIK (NP_180131.3),Citation44 AKT1 (NP_180233.1),Citation39 KAT1 (NP_199436.1),Citation45 KAT2 (NP_193563.3),Citation46 AKT2 (NP_567651.1),Citation47 AtKC1 (NP_974665.1),Citation10 SKOR (NP_186934.1)Citation48 and GORK (NP_198566.2)Citation49), 3 from grapevine (VvK1.1 (CAZ64538.1),Citation50 VvK1.2 (NP_001268010.1)Citation51 and VvSIRK (NP_001268073.1)Citation52), one from potato (SKT1 (NP_001275347.1)Citation10), one from maize (ZmK2 (NP_001105120.1)Citation53) and one from carrot (Kdc1 (CAB62555.1)Citation54). (E) Mutation of the conserved residues Tyr193 and Arg197 into Ala renders KAT2 channels electrically silent in Xenopus oocytes. Representative current traces recorded by 2-electrode voltage-clamp recordings in occytes injected with (from left to right): water, KAT2, KAT2 Y193A and KAT2 R197A in 100 mM K+ bath solution. Applied activation membrane voltages ranged from +40 to −170 mV (increments of 15 mV; holding potential, 0 mV; deactivation potential, −40 mV). Site-directed mutagenesis, cRNA preparation and solutions are described in Nieves-Cordones et al. 2014.Citation9
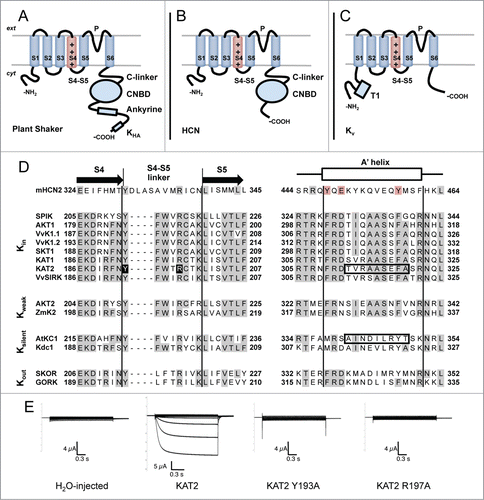
Plant Shaker channels belong to the 6 transmembrane-1 pore (6TM-1P) cation channel superfamily where we also find the well-characterized animal Shaker (Kv) and HCN channels.Citation7 The latter channels, which regulate action potentials in cardiac muscle cells and neurons, are the closest homologues of plant Shaker channels in this channel superfamily.Citation9,14,15 HCN channels share with plant Shaker channels the subunit structure described above with the exception of the distal cytoplasmic part that differs (ankyrin and KHA domains in plant Shaker channels and extreme C-terminal part in HCNs) ().Citation9,14 At the functional level, HCN channels exhibit inward-rectification and are opened by hyperpolarized membrane potentials, like plant Shaker K+ inward-rectifiers. On the other hand, animal Kv K+ channels activate at depolarized membrane voltages (),Citation16 as in the case of plant Shaker outwardly-rectifying K+ channels. In both HCNs and plant Shaker channels, the cytosolic C-terminal part participate in tetramer formationCitation13,14,17 while for Kv channels, it is the N-terminal tail that is involved in this process.Citation18 1 common point among all these type of channels is that the voltage sensor (S4) moves in a similar fashion with voltage, thus the type of rectification exhibited by the channel depends on how such movement of S4 is coupled to pore opening and closing.Citation5,14 With this regard, plant Shaker channels are an excellent model to study the mechanism of voltage-gating since inwardly-, weakly- and outwardly-rectifying channels exhibit different voltage sensitivity but very similar subunit structure. Several studies have addressed this issue by using chimeric and random mutated channel Shaker subunits.Citation19-23 Indeed, in the S4-S6 domains of Arabidopsis SKOR outwardly-rectifying K+ channel and KAT1 inwardly-rectifying K+ channel, different zones were deeply investigated. The results obtained so far, revealed that the N-terminal part of the S5 and the C-terminal part of S6 transmembrane helices are key zones that influence channel gating.Citation22,23
Channel Opening in Animal HCN Channels Needs Conformational Changes in the Pore-Forming Module (S5-P-S6) and in the C-Terminus Region
In the inward-rectifying HCN channels, where considerable amount of data at the structural level is available including structure-function relationship by crystallography,Citation15,24,25 both C-linker and CNBD significantly contribute to voltage-gating. Following the end of the last transmembrane segment (S6), the C-linker domain consists of 6 α-helices which are named A′ to F′ according to their position after the S6 segment. Downstream the C-linker domain, the CNBD includes 4 α-helices (A, P, B, C) and a β-roll between the A- and B-helices with a jelly-roll-like topology. Binding site of cyclic-nucleotides is located inside the β-roll.Citation24 Crystallographic and functional information obtained in HCN channels showed that the C-linker domain exerts an inhibitory effect on channel opening that is waived by the binding of cyclic-nucleotides by the CNBD.Citation24,26 In this context, channel opening requires, in addition to the rearrangements necessary to set the pore in the open conformation, a displacement of the C-linker domain, especially of its first α-helix (A′) which is parallel to the membrane, located just below the channel transmembrane pore and in close proximity to the S4-S5 loop. Such displacement takes place through interactions of S4-S5 linker residues with the A′ helix residues of the same subunit or of neighboring subunits.Citation27,28 Indeed, the HCN model for voltage-gating implies that S4 movements induced by membrane polarization lead to a series of conformational changes in the pore-forming module (S5-P-S6) and in the C-terminus (C-linker and CNBD domains) as well.
Contribution of the S4-S5 Linker in Plant Shaker Channel Functionality
In HCN channels, Alanine-scanning mutagenesis of the S4–S5 linker was used to identify different residues that are important for the channel functionality. Indeed it has been demonstrated that 2 amino acids of the HCN2 S4-S5 linker, Y331, and R339, disturbed normal channel closing when they are mutated in Ala.Citation29 The authors concluded that S4-S5 linker mediates the coupling between voltage sensing and pore movements. In plant KAT2 Shaker subunit, corresponding amino acids are Y193 and R197 () and we showed here that when mutated in Ala, the mutated channel gave rise to a non-functional channel in Xenopus oocytes (). This strongly suggested a similar role of the S4-S5 linker for the coupling of voltage sensor movements to channel opening and closing, in both HCN and plant Shaker channels.
Contribution of the C-Linker in Plant Shaker Channel Functionality
If we take into consideration information gained in Arabidopsis Shaker weakly- and inwardly-rectifying channels with respect the involvement of the N-terminal part of the C-linker, striking similarities with HCN channels can be also found at this level. AKT2 forms weakly-rectifying K+ channels in Xenopus oocytes and in COS cells which displays 2 type of current components: one corresponds to channels behaving like K+ selective inwardly-rectifying channels (mode 1) and the other to channels producing K+ selective leak-like currents (mode 2).Citation30 It is known that switching between these 2 gating modes is under a phosphorylation control.Citation31 Two crucial phosphorylation sites, S210 and S329, located in the S4-S5 linker and in the C-linker A′ helix, respectively, have been pinpointed and studied by site-directed mutagenesis. Indeed the double-Asn mutation (S210N-S329N mutant) which seemed able to mimic phosphorylated serines,Citation32 irreversibly set the channels in mode 2 while the double-Ala mutation which mimics a non-phosphorylated stateCitation33 made the channel gate in mode 1.Citation31 Interestingly, certain mutations in the S4-S5 linker and in the A′ helix of HCN channels also leads to channels with an increased leak current component.Citation27,29
In the case of Arabidopsis inward-rectifiers, data has been obtained from the twin subunits KAT1 and KAT2 by different approaches. KAT1 and KAT2 channels are known to be the major inwardly rectifying K+ channels in guard cells and play a key role in stomatal opening.Citation34,35 In guard cells, the activity of KAT1 channel is likely to be regulated by phosphorylation via the SnRK2/SnRK2.6 kinase OST1 (Open Stomata 1).Citation36 When looking for target sites of the OST1 protein kinase in the C-terminal part of KAT1 subunit, 2 Thr residues (Thr306 and Thr308) in the N-terminal part of the C-linker domain, just before the beginning of the A′ helix, have been identified.Citation37 Functional analyses of such residues revealed a dramatic effect of the mutations performed in Thr306 which gave rise to a non-functional channel in Xenopus oocytes and in yeasts. Although the impact of such mutations on channel traffic was not assessed, the authors proposed that Thr306 mutants were affected in channel gating. More recently, Nieves-Cordones et al. (2014)Citation9 showed that the region homologous to the A′ helix of HCN channels plays an important role in KAT2 channel functionality. In this report, a KAT2-AtKC1 chimeric channel, in which KAT2 C-linker domain was replaced to a great extent by that of AtKC1, was targeted to the plasma membrane but remained electrically silent in Xenopus oocytes. Such silent chimeric channel was functionally rescued by the presence of the native KAT2 9 aminoacid stretch 312TVRAASEFA320 covering the A′ helix (). Moreover, the native KAT2 subunit was rendered inactive in the range of membrane potentials applicable in Xenopus oocytes by replacing such KAT2 9 aminoacid stretch by the corresponding sequence of AtKC1 (341AINDILRYT349; ).
Moreover, it has been shown that high-affinity metal bridges made between the S4-S5 linker and the A′ helix of a HCN channel can block the channel either in a closed state (as in the case of KAT1 or KAT2 mutants) or in an open (leak) state (as in the case of AKT2 mode 2) implying that these regions move relative to each other during gating.Citation28 Thus, in absence of plant Shaker crystallographic data, it is tempting to speculate that the same voltage-gating mechanism described for HCN channels could be applied to plant Shaker inwardly- and weakly-rectifying K+ channels: S4 drives the C-linker relative position through the S4-S5 linker. In contrast, no functional information concerning the role of S4-S5 linker and the putative A′ helix is available on plant outward-rectifying K+ channels, but protein alignment shows that such regions significantly diverge among functional groups of plant Shaker channels and could constitute additional elements for outward rectification ().
Plant Shaker CNBD and Binding of Cyclic-Nucleotides
Contrary to HCN channels, plant Shaker channels do not seem to be regulated by cyclic nucleotides since they have little or no effect on K+ conductance.Citation38,39 Such lack of effect is not surprising as the key residues involved in cyclic-nucleotide binding are not conserved in plant Shaker channels.Citation24 However, deletions in the CNBD of KAT1 and SKOR lead to non-functional channels in Xenopus oocytes and clearly showed that this domain is important for channel functionality.Citation13,40 A mechanism has been described in animal ELK (ether-à-go-go like K+ channel) channels, another member of the large 6 transmembrane-1 pore (6TM-1P) cation channel superfamily, to explain the inability of its CNBD to respond to cyclic-nucleotides. Indeed, crystal structure of the ELK channel from zebrafish (zELK) revealed that the CNBD binding pocket is occupied by a β-strand that acts as an intrinsic ligand.Citation41 Again, crystal structure of a plant Shaker channel would provide a precise answer about the conformation of the CNBD and help us to understand whether a similar mechanism exist for plant Shaker channels. Interestingly, the Arabidopsis cyclic-nucleotide gated channel 18 (AtCNGC18), which also possesses a CNBD on its C-terminus, seems to respond to cyclic-nucleotides.Citation42 Thus, CNGC's could constitute a more interesting candidate for cyclic-nucleotide-mediated regulation in plant cells rather than Shaker channels. Further work aiming at analyzing the CNBD's from plant Shaker and CNGC channels would help to understand the molecular basis for cyclic-nucleotide binding in plant channels.
Conclusions
Functional diversity and straightforward expression in heterologous systems of plant Shaker channels has permitted the identification of distinct residues involved in gating-specific features. As a result, data obtained in plant Shaker inwardly- and weakly-rectifying channels suggests that a very similar voltage-gating mechanism to that of HCN channels may be operating in plant Shaker channels in which membrane polarization is perceived by the S4 and subsequent conformational changes at the pore level and in the C-terminus, particularly in the putative A′-helix of the C-linker domain, allow the channel to open and close. However, it remains to be assessed whether this mechanism is also present in plant Shaker outwardly-rectifying channels. Finally, analysis of crystal structures of plant Shaker channels will definitely provide us with critical information, unavailable at this point, about the residues and conformational changes taking place during channel gating and the likely insensitivity of their CNBD to cyclic-nucleotides.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Very AA, Sentenac H. Molecular mechanisms and regulation of K +transport in higher plants. Annu Rev Plant Biol 2003; 54:575-603; PMID:14503004; http://dx.doi.org/10.1146annurev.arplant.54.031902.134831
- Lebaudy A, Very AA, Sentenac H. K+ channel activity in plants: genes, regulations and functions. FEBS Lett 2007; 581:2357-66; PMID:17418142; http://dx.doi.org/10.1016j.febslet.2007.03.058
- Wang Y, Wu WH. Potassium transport and signaling in higher plants. Annu Rev Plant Biol 2013; 64:451-76; PMID:23330792; http://dx.doi.org/10.1146annurev-arplant-050312-120153
- Sharma T, Dreyer I, Riedelsberger J. The role of K(+) channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front Plant Sci 2013; 4:224; PMID:23818893; http://dx.doi.org/10.3389fpls.2013.00224
- Dreyer I, Blatt MR. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci 2009; 14:383-90; PMID:19540150; http://dx.doi.org/10.1016j.tplants.2009.04.001
- Ehrhardt T, Zimmermann S, Mueller-Roeber B. Association of plant K+ (in) channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett 1997; 409:166-70; PMID:9202139
- Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H. Five-group distribution of the Shaker-like K+ channel family in higher plants. J Mol Evol 2003; 56:418-34; PMID:12664162; http://dx.doi.org/10.1007s00239-002-2413-2
- Very AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol 2014; 171:748-69; PMID:24666983; http://dx.doi.org/10.1016j.jplph.2014.01.011
- Nieves-Cordones M, Chavanieu A, Jeanguenin L, Alcon C, Szponarski W, Estaran S, Cherel I, Zimmermann S, Sentenac H, Gaillard I. Distinct amino acids in the C-linker domain of the Arabidopsis K+ channel KAT2 determine its subcellular localization and activity at the plasma membrane. Plant Physiol 2014; 164:1415-29; PMID:24406792; http://dx.doi.org/10.1104pp.113.229757
- Dreyer I, Antunes S, Hoshi T, Mueller-Roeber B, Palme K, Pongs O, Reintanz B, Hedrich R. Plant K+ channel alpha-subunits assemble indiscriminately. Biophys J 1997; 72:2143-50; PMID:9129816; http://dx.doi.org/10.1016S0006-3495(97)78857-X
- Lebaudy A, Hosy E, Simonneau T, Sentenac H, Thibaud JB, Dreyer I. Heteromeric K+ channels in plants. Plant J 2008; 54:1076-82; PMID:18346194; http://dx.doi.org/10.1111j.1365-313X.2008.03479.x
- Dreyer I, Uozumi N. Potassium channels in plant cells. FEBS J 2011; 278:4293-303; PMID:21955642; http://dx.doi.org/10.1111j.1742-4658.2011.08371.x
- Dreyer I, Poree F, Schneider A, Mittelstadt J, Bertl A, Sentenac H, Thibaud JB, Mueller-Roeber B. Assembly of plant Shaker-like K(out) channels requires two distinct sites of the channel alpha-subunit. Biophys J 2004; 87:858-72; PMID:15298894; http://dx.doi.org/10.1529biophysj.103.037671
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol 2006; 68:375-401; PMID:16460277; http://dx.doi.org/10.1146annurev.physiol.68.040104.134728
- Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 2009; 66:470-94; PMID:18953682; http://dx.doi.org/10.1007s00018-008-8525-0
- Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 2007; 450:376-82; PMID:18004376; http://dx.doi.org/10.1038nature06265
- Daram P, Urbach S, Gaymard F, Sentenac H, Cherel I. Tetramerization of the AKT1 plant potassium channel involves its C-terminal cytoplasmic domain. EMBO J 1997; 16:3455-63; PMID:9218788; http://dx.doi.org/10.1093emboj16.12.3455
- Strang C, Cushman SJ, DeRubeis D, Peterson D, Pfaffinger PJ. A central role for the T1 domain in voltage-gated potassium channel formation and function. J Biol Chem 2001; 276:28493-502; PMID:11312262; http://dx.doi.org/10.1074jbc.M010540200
- Poree F, Wulfetange K, Naso A, Carpaneto A, Roller A, Natura G, Bertl A, Sentenac H, Thibaud JB, Dreyer I. Plant K(in) and K(out) channels: approaching the trait of opposite rectification by analyzing more than 250 KAT1-SKOR chimeras. Biochem Biophys Res Commun 2005; 332:465-73; PMID:15894288; http://dx.doi.org/10.1016j.bbrc.2005.04.150
- Michard E, Lacombe B, Poree F, Mueller-Roeber B, Sentenac H, Thibaud JB, Dreyer I. A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J Gen Physiol 2005; 126:605-17; PMID:16316977; http://dx.doi.org/10.1085jgp.200509413
- Li L, Liu K, Hu Y, Li D, Luan S. Single mutations convert an outward K+ channel into an inward K+ channel. Proc Natl Acad Sci U S A 2008; 105:2871-6; PMID:18287042; http://dx.doi.org/10.1073pnas.0712349105
- Gajdanowicz P, Garcia-Mata C, Gonzalez W, Morales-Navarro SE, Sharma T, Gonzalez-Nilo FD, Gutowicz J, Mueller-Roeber B, Blatt MR, Dreyer I. Distinct roles of the last transmembrane domain in controlling Arabidopsis K +channel activity. New Phytol 2009; 182:380-91; PMID:19192193; http://dx.doi.org/10.1111j.1469-8137.2008.02749.x
- Riedelsberger J, Sharma T, Gonzalez W, Gajdanowicz P, Morales-Navarro SE, Garcia-Mata C, Mueller-Roeber B, Gonzalez-Nilo FD, Blatt MR, Dreyer I. Distributed structures underlie gating differences between the kin channel KAT1 and the Kout channel SKOR. Mol Plant 2010; 3:236-45; PMID:20007672; http://dx.doi.org/10.1093mpssp096
- Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 2003; 425:200-5; PMID:12968185; http://dx.doi.org/10.1038nature01922
- Flynn GE, Zagotta WN. Molecular mechanism underlying phosphatidylinositol 4,5-bisphosphate-induced inhibition of SpIH channels. J Biol Chem 2011; 286:15535-42; PMID:21383006; http://dx.doi.org/10.1074jbc.M110.214650
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 2001; 411:805-10; PMID:11459060; http://dx.doi.org/10.103835081088
- Decher N, Chen J, Sanguinetti MC. Voltage-dependent gating of hyperpolarization-activated, cyclic nucleotide-gated pacemaker channels: molecular coupling between the S4-S5 and C-linkers. J Biol Chem 2004; 279:13859-65; PMID:14726518; http://dx.doi.org/10.1074jbc.M313704200
- Kwan DC, Prole DL, Yellen G. Structural changes during HCN channel gating defined by high affinity metal bridges. J Gen Physiol 2012; 140:279-91; PMID:22930802; http://dx.doi.org/10.1085jgp.201210838
- Chen J, Mitcheson JS, Tristani-Firouzi M, Lin M, Sanguinetti MC. The S4-S5 linker couples voltage sensing and activation of pacemaker channels. Proc Natl Acad Sci U S A 2001; 98:11277-82; PMID:11553787; http://dx.doi.org/10.1073pnas.201250598
- Dreyer I, Michard E, Lacombe B, Thibaud JB. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett 2001; 505:233-9; PMID:11566182
- Michard E, Dreyer I, Lacombe B, Sentenac H, Thibaud JB. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J 2005; 44:783-97; PMID:16297070; http://dx.doi.org/10.1111j.1365-313X.2005.02566.x
- Pearlman SM, Serber Z, Ferrell JEJ. A mechanism for the evolution of phosphorylation sites. Cell 2011; 147:934-46; PMID:22078888; http://dx.doi.org/10.1016j.cell.2011.08.052
- Wang ZY, Wang F, Sellers JR, Korn ED, Hammer JAr. Analysis of the regulatory phosphorylation site in Acanthamoeba myosin IC by using site-directed mutagenesis. Proc Natl Acad Sci U S A 1998; 95:15200-5; PMID:9860946
- Lebaudy A, Pascaud F, Very AA, Alcon C, Dreyer I, Thibaud JB, Lacombe B. Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J Biol Chem 2010; 285:6265-74; PMID:20040603; http://dx.doi.org/10.1074jbc.M109.068445
- Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB, Very AA, Simonneau T, Sentenac H. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc Natl Acad Sci U S A 2008; 105:5271-6; PMID:18367672; http://dx.doi.org/10.1073pnas.0709732105
- Acharya BR, Jeon BW, Zhang W, Assmann SM. Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol 2013; 200:1049-63; PMID:24033256; http://dx.doi.org/10.1111nph.12469
- Sato A, Sato Y, Fukao Y, Fujiwara M, Umezawa T, Shinozaki K, Hibi T, Taniguchi M, Miyake H, Goto DB, et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2OST1SnRK2.6 protein kinase. Biochem J 2009; 424:439-48; PMID:19785574; http://dx.doi.org/10.1042BJ20091221
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol 1995; 105:309-28; PMID:7769379
- Gaymard F, Cerutti M, Horeau C, Lemaillet G, Urbach S, Ravallec M, Devauchelle G, Sentenac H, Thibaud JB. The baculovirusinsect cell system as an alternative to Xenopus oocytes. First characterization of the AKT1 K+ channel from Arabidopsis thaliana. J Biol Chem 1996; 271:22863-70; PMID:8798465
- Marten I, Hoshi T. Voltage-dependent gating characteristics of the K+ channel KAT1 depend on the N and C termini. Proc Natl Acad Sci U S A 1997; 94:3448-53; PMID:9096414
- Brelidze TI, Carlson AE, Sankaran B, Zagotta WN. Structure of the carboxy-terminal region of a KCNH channel. Nature 2012; 481:530-3; PMID:22230959; http://dx.doi.org/10.1038nature10735
- Gao QF, Fei CF, Dong JY, Gu LL, Wang YF. Arabidopsis CNGC18 is a Ca(2)(+)-permeable channel. Mol Plant 2014; 7:739-43; PMID:24380879; http://dx.doi.org/10.1093mpsst174
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature 1998; 393:587-91; PMID:9634236; http://dx.doi.org/10.103831255
- Mouline K, Very AA, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud JB, Sentenac H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K(+) channel in Arabidopsis. Genes Dev 2002; 16:339-50; PMID:11825875; http://dx.doi.org/10.1101gad.213902
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 1992; 258:1654-8; PMID:8966547
- Pilot G, Lacombe B, Gaymard F, Cherel I, Boucherez J, Thibaud JB, Sentenac H. Guard cell inward K+ channel activity in arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J Biol Chem 2001; 276:3215-21; PMID:11042178; http://dx.doi.org/10.1074jbc.M007303200
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB. A shaker-like K(+) channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 2000; 12:837-51; PMID:10852932
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998; 94:647-55; PMID:9741629
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett 2000; 486:93-8; PMID:11113445
- Cuellar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I. A grapevine Shaker inward K(+) channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 2010; 61:58-69; PMID:19781051; http://dx.doi.org/10.1111j.1365-313X.2009.04029.x
- Cuellar T, Azeem F, Andrianteranagna M, Pascaud F, Verdeil JL, Sentenac H, Zimmermann S, Gaillard I. Potassium transport in developing fleshy fruits: the grapevine inward K(+) channel VvK1.2 is activated by CIPK-CBL complexes and induced in ripening berry flesh cells. Plant J 2013; 73:1006-18; PMID:23217029; http://dx.doi.org/10.1111tpj.12092
- Pratelli R, Lacombe B, Torregrosa L, Gaymard F, Romieu C, Thibaud JB, Sentenac H. A grapevine gene encoding a guard cell K(+) channel displays developmental regulation in the grapevine berry. Plant Physiol 2002; 128:564-77; PMID:11842160; http://dx.doi.org/10.1104pp.010529
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci U S A 1999; 96:12186-91; PMID:10518597
- Naso A, Montisci R, Gambale F, Picco C. Stoichiometry studies reveal functional properties of KDC1 in plant shaker potassium channels. Biophys J 2006; 91:3673-83; PMID:16920836; http://dx.doi.org/10.1529biophysj.106.091777
