Abstract
Using a cuvette for simultaneous measurement of net photosynthesis in above ground plant organs and root respiration we investigated the effect of reduced leaf glucokinase activity on plant carbon balance. The gin2–1 mutant of Arabidopsis thaliana is characterized by a 50% reduction of glucokinase activity in the shoot, while activity in roots is about fivefold higher and similar to wild type plants. High levels of sucrose accumulating in leaves during the light period correlated with elevated root respiration in gin2–1. Despite substantial respiratory losses in roots, growth retardation was moderate, probably because photosynthetic carbon fixation was simultaneously elevated in gin2–1. Our data indicate that futile cycling of sucrose in shoots exerts a reduction on net CO2 gain, but this is over-compensated by the prevention of exaggerated root respiration resulting from high sucrose concentration in leaf tissue.
Abbrevations
| FW | = | fresh weight |
| gin2–1 | = | knockout mutant of hexokinase1 |
| NPS | = | net photosynthesis |
| SEM | = | standard error of mean |
| WT | = | wild type |
Assessment of plant-environment carbon dioxide relations is hampered by the difficulties to discriminate photosynthetic CO2 fixation from respiratory release in the above-ground organsCitation1 on the one hand and methodological restrictions to measure root respiration in soil grown plants on the other hand. The contribution of root-derived CO2 efflux from soils is frequently assessed by root exclusion techniques, shading or other methods that reduce the plant-associated component. However, all these methods have the disadvantage of severely interfering with the system under investigation. Alternative methods based on stable or radioactive carbon isotopes are less invasive, but require complex and costly equipment and, because they are mostly based on pulse labeling strategies, are not suitable for long term observations.Citation2
For the quantification of root respiration over full diurnal cycles, hydroponic systems have been employed that use aerated nutrient solutions and infrared analysis of CO2 in the off-gasCitation3,4 or enclosed systems, in which oxygen consumption is measured using Clark type electrodes or similar techniques.Citation5,6 While the latter bears the risk of exerting a substrate limitation on respiration, the former has a rather low time resolution because of the slow equilibration of CO2 concentration in aqueous and gas phase.
We analyzed whole plant CO2 exchange with the environment using a recently developed device that allows simultaneous measurement of net photosynthesis (NPS) in green organs and respiration of roots kept in an aeroponic environment.Citation7 Aeroponic measurement of root respiration combines the advantages of non-invasiveness with fast time response and high sensitivity of gas phase measurements of CO2 based on infrared absorption.
Following an analysis of plant-environment CO2 exchange in the starchless pgm mutant of ArabidopsisCitation7 we extended our investigation to the gin2–1 knockout mutant of hexokinase 1 (At4g29130). This mutant has a strongly reduced glucokinase activity that results in sensitivity to high light and altered hormone responses.Citation8 Besides its function as a glucose sensor, glucokinase is a key enzyme in re-synthesis of sucrose hydrolyzed by invertase,Citation9 which has been termed “futile sucrose cycling” and is believed to be important in stabilizing the carbohydrate status of leaf tissues.Citation10,11
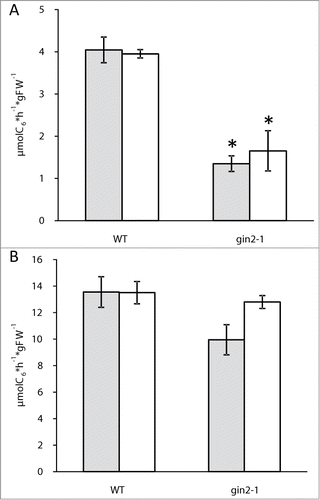
For soil-grown plants a 50% reduction in glucokinase activity in leaf tissues has been reported for the gin2–1 mutant grown under various light conditions.Citation8 We observed the same reduction in leaves of hydroponically cultivated plants using the method of Wiese et al.,Citation12 which is based on the oxidation of glucose-6-phosphate to 6-phosphogluconolactone (). In contrast to leaves, roots of wild type plants had a threefold higher glucokinase activity as compared to leaves, and there was no reduction in the gin2–1 mutant (), indicating that an enzyme not encoded by the HXK1 gene is dominating in root tissue. It can therefore be concluded that at least the metabolic consequences of the gin2–1 mutation affect only shoot but not root tissues.
Figure 1. Maximum enzymatic glucose phosphorylation in μmol C6 per hour and gFW of shoot (A) and root (B) immediately before the end of the night (gray) and the end of the day (white). Results represent means with SEMs (n = 5). Group means varying significantly at the 5% level were marked with asterisk. Plants were cultivated hydroponically under long day conditions (16 h light, intensity: 150 μmol m−2 s−1, 22°C; 8 h dark, 16°C) for 7 days, following cultivation under short day conditions (8 h light, intensity: 150 μmol m−2 s−1, 22°C; 16 h dark, 16°C) for 3 weeks. Seedlings were brought into hydroponic culture 21 d after sowing in soil (GS20) / vermiculite (1:1) applying short day conditions.
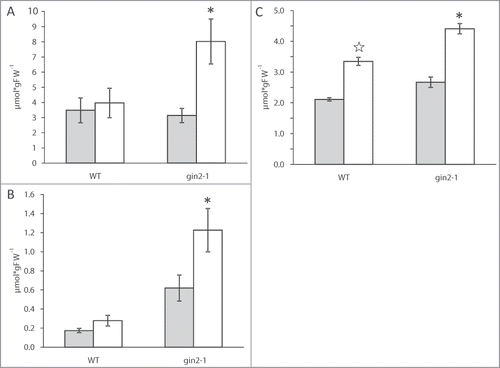
Concentrations of soluble sugars were measured at the end of the light and the end of the dark period following the method described in Brauner et al.Citation7 Glucose was the most abundant soluble sugar in wild type (WT) and gin2–1 at both time points and accumulated during the day in leaves of gin2–1 but not in WT (). Higher glucose than sucrose levels are typical of Arabidopsis plants grown under high light intensities and long day conditions. The accumulation of hexoses points to a high rate of operation for sucrose cycling, which is limited by hexokinase activity.Citation9 At the end of the day, glucose levels in gin2–1 were twice as high as in WT, clearly pointing to a limitation in re-entering hexoses, produced by hydrolysis of sucrose, into the carbohydrate metabolic network of the mutant. Although at much lower levels, fructose followed the same pattern as glucose with an increase during the light phase in gin2–1 (). Sucrose accumulated during the light phase in WT as well as in the gin2–1 mutant, but the increase was stronger in gin2–1, thus leading to significantly higher sucrose levels in the mutant at the end of the day ().
Figure 2. Concentrations of glucose (A), fructose (B) and sucrose (C) in leaf tissues of Arabidopsis at the end of the night (gray) and the end of the day (white). Results represent means with SEMs (n = 5). Group means varying significantly at the 5% level were marked with different asterisks.
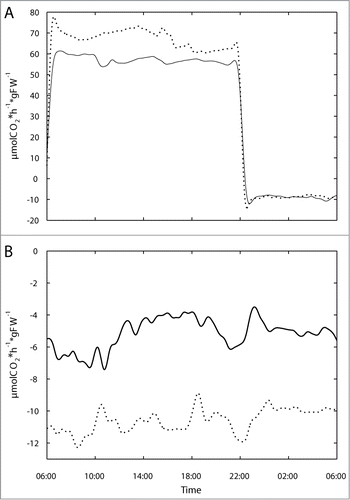
Because we have previously found stimulation of root respiration in the starchless pgm mutant that has elevated sugar concentrations in leaves,Citation7 we measured NPS of shoots and respiration of roots of gin2–1 and WT. Profiles of these measurements are shown in . It can be seen that, on a fresh weight basis, gin2–1 displayed a 20% increase in NPS of shoots during the light phase, and nearly a doubling of root respiration over the entire diurnal cycle. Respiration of the shoot during the night was unaltered. At the whole plant level WT lost 28% of NPS by respiration while gin2–1 lost 37%. This raised carbon deficit was reflected in a 23% reduced total biomass formation after the 50 day growth phase (WT: 1.22 ± 0.11 gFW; gin2–1: 0.94 ± 0.08 gFW; n = 10). Both genotypes displayed a root-to-shoot ratio of 0.33. Considering the strong stimulation of root respiration, the reduction in biomass was much lower than in the starchless pgm mutant, which had a similarly increased root respiration under the experimental conditions described in Brauner et al.Citation7 The most obvious explanation for this finding is a compensation of respiratory losses of the root by increased daily fixation in the shoot, and this could very likely be caused by a reduction of futile cycling of sucrose. Hydrolysis of sucrose by vacuolar and / or neutral invertases and the consecutive phosphorylation of hexoses by hexokinases that precedes re-synthesis of sucrose amounts to about 25% of sucrose turnover in Arabidopsis leavesCitation11 and creates a significant energy sink, thus reducing photosynthetic efficiency. However, sucrose cycling serves to stabilize the sucrose concentration in leaf mesophyll cells,Citation10 and the biomass reduction in the gin2–1 mutant demonstrates that the costs for this stabilizing effect are over-compensated by the prevention of increased root respiration that would otherwise result from sucrose accumulation in leaf cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Katrin Brauner was supported by a fellowship granted by the Foundation of German Business (Stiftung der Deutschen Wirtschaft).
References
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham JR. Ratcliffe RG, Sweetlove LJ, Fernie AR. Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 2007; 50:1093-106; PMID:17461782; http://dx.doi.org/10.1111/j.1365-313X.2007.03115.x
- Werth M, Kuzyakov Y. Three-source partitioning of CO2 efflux from maize field soil by 13C natural abundance. Plant Nutr Soil Sci 2009; 172:487-99; http://dx.doi.org/10.1002/jpln.200700085
- Scheurwater I, Cornelissen C, Dictus F, Welschen F, Lambers H. Why do fast- and slow-growing grass species differ so little in their rate of root respiration, considering the large differences in rate of growth and ion uptake? Plant Cell Environ 1998; 21:995-1005; http://dx.doi.org/10.1046/j.1365-3040.1998.00341.x
- Zeeman SC, ap Rees T. Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ 1999; 22:1445-53; http://dx.doi.org/10.1046/j.1365-3040.1999.00503.x
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 2000; 381:723-40; PMID:11030430; http://dx.doi.org/10.1515/BC.2000.093
- Rachmilevitcha R, Xua Y, Gonzalez-Melerb MA, Huanga B, Lambers H. Cytochrome and alternative pathway activity in roots of thermal and non-thermal Agrostis species in response to high soil temperature. Physiol Plant 2007; 129:163-74; http://dx.doi.org/10.1111/j.1399-3054.2006.00784.x
- Brauner K, Hörmiller I, Nägele T, Heyer AG. Exaggerated root respiration accounts for growth retardation in a starchless mutant of Arabidopsis thaliana. Plant J 2014; 79:82-91; PMID:24836712; http://dx.doi.org/10.1111/tpj.12555
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003; 300: 332-336; PMID:12690200; http://dx.doi.org/10.1126/science.1080585
- Henkel S, Nägele T, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG. A systems biology approach to analyse leaf carbohydrate metabolism in Arabidopsis thaliana. EURASIP J Bioinf Syst Biol 2011; 2.
- Huber S. Biochemical mechanism for regulation of sucrose accumulation in leaves during photosynthesis. Plant Physiol 1989; 91:656-62; PMID:16667083; http://dx.doi.org/10.1104/pp.91.2.656
- Nägele T, Henkel S, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG. Mathematical modeling of the central carbohydrate metabolism in Arabidopsis reveals a substantial regulatory influence of vacuolar invertase on whole plant carbon metabolism. Plant Physiol 2010; 153:260-72; http://dx.doi.org/10.1104/pp.110.154443
- Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge U, Weber A. Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett. 1999; 461:13-8; PMID:10561488; http://dx.doi.org/10.1016/S0014-5793(99)01417-9