Abstract
Bidirectional nutrient transfer is one of the key features of the arbuscular mycorrhizal symbiosis. Recently we were able to identify a Medicago truncatula mutant (mtha1-2) that is defective in the uptake of phosphate from the periarbuscular space due to a lack of the energy providing proton gradient provided by the symbiosis specific proton ATPase MtHA11 In order to further characterize the impact of fungal colonization on the plant metabolic status, without the beneficial aspect of improved mineral nutrition, we performed leaf ion analyses in mutant and wildtype plants with and without fungal colonization. Although frequency of fungal colonization was unaltered, the mutant did not show a positive growth response to mycorrhizal colonization. This indicates that nutrient transfer into the plant cell fails in the truncated arbuscules due to lacking expression of a functional MtHA1 protein. The leaves of wildtype plants showed clear metabolic responses to root mycorrhizal colonization, whereas no changes of leaf metabolite levels of mycorrhizal mtha1-2 plants were detected, even though they were colonized. These results show that MtHa1 is indispensable for a functional mycorrhizal symbiosis and, moreover, suggest that fungal root colonization per se does not depend on nutrient transfer to the plant host.
Results
Plants respond to mycorrhizal colonization by changes of the metabolome, roots of Medicago truncatula show clear responses to mycorrhizal colonization.Citation2 The changes seem to be species-specific, with Medicago truncatula showing a more pronounced metabolic answer than other dicots, probably due to a higher symbiotic capability.Citation3 The most prominent changes are visible in the catabolic and amino acid metabolism, probably due to the improved carbon sink strength and an improved P- and N-level.Citation4 However, not only P and N, but also S provided by the symbiotic uptake pathway leads to changes in the metabolic response.Citation5 The recent identification of M. truncatula mutants defective in the symbiotic transfer of nutrients across the periarbuscular space allows us now to study the impact of fungal colonization independent of the improved nutrition of the plant.Citation1,6
The lack of functional MtHA1 proteins is leading to the incapability of phosphate uptake, and presumably also other ions, from the periarbuscular space. In accordance with this assumption, we did not observe a positive growth response of mycorrhizal mtha1-2 mutant plants. However, fungal colonization accompanied with intercellular hyphal growth was still possible, indicating that the fungus is still able to get carbon from the mutant plants. To investigate the impact of this carbon drain on the metabolism of mtha1-2 mutants we analyzed growth parameters and leaf ion contents of mutants and wildtype plants. In order to investigate the impact of carbon drain under varying carbon and phosphate conditions, we compared plants grown under 2 different phosphate regimes (1 mM and 20 μM) and either under standard (16 h light/8 h dark) or under short day condition (12 h light/12 h dark). All plants were first grown at standard conditions for 2 weeks followed by a 26-day growth period at either standard light or short day conditions. An analysis of root and shoot fresh weights showed that phosphate nutrition significantly influenced shoot fresh weights (ANOVA, P < 0.001) and that mycorrhizal colonization significantly influenced shoot fresh weights under low phosphate depending on the plant genotype (ANOVA, P < 0.039). Shoot growth was limited by low phosphate as well as by short days, with shoot weights of mycorrhizal wildtype plants being significantly increased as compared to non-mycorrhizal plants or mutants due to mycorrhizal nutrient uptake. This was also true under short day conditions, indicating that the mycorrhizal nutrient uptake is still beneficial for plant growth and that the carbon transfer to the fungus is not growth limiting. The carbon shortage by short day treatment resulted in reduced root fresh weights at moderate phosphate condition (), which represents the condition of most drastic change in the plant carbon to phosphate (C/P) ratio. Root fresh weights were not significantly reduced by short day treatment when plants were grown under low phosphate regime (). Mycorrhizal colonization at low phosphate fertilization results in significantly reduced C/P ratio due to mycorrhizal phosphate uptake. As a consequence, we found increased shoot growth and reduced root growth of mycorrhizal plants at low phosphate.
Figure 1. Metabolic responses of Medicago truncatula wildtype and mtha1-2 mutant plants to mycorrhization, phosphate supply and day length. Ion concentrations in leaves were analyzed from leaf samples of 5-week-old plants, grown for 3 weeks under the stated conditions. The plots were applied for contents of 6 ions (phosphate, sulfate, nitrate, citrate, fumarate and chloride) and root and shoot fresh weight per plant. Data shown are averages of 3 biological replicates. (A) Principal component analysis (PCA) of ion contents and fresh weight data. Triangles represent wildtype plants, squares represent mtha1-2 mutant plants. Filled symbols represent mycorrhizal plants. Yellow: plants were grown at strong phosphate starvation (20 μM) and standard light regime (16/8 h), red: plants were grown at strong phosphate starvation and short day condition (12/12 h), green: plants were grown at moderate phosphate starvation (1 mM) and short day condition, blue: plants were grown at moderate phosphate starvation and standard light regime. (B) Clustering of ion content and fresh weight data showed that values derived from mycorrhizal mtha1-2 plants (m_myc) group with data from non-mycorrhizal plants (w_nm or m_nm), whereas mycorrhizal wildtype values (w_myc) are clearly separated from all other samples. This is independent of P nutrition (_−P or _+P) and day length (_dark = short day conditions). Data were normalized on the average of the values obtained from all samples. The data with log2 scale were submitted to Hierarchical Clustering Analysis (HCA) using Pearson correlation to identify clusters of similar profiles.
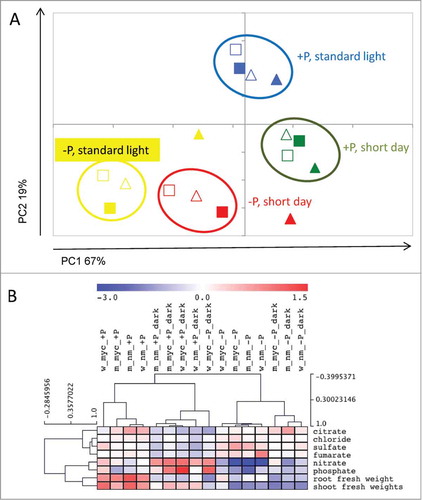
To investigate the impact of mycorrhizal nutrient uptake on plant nutrition, we analyzed leaf ion levels. Anions were analyzed by high-performance anion-exchange chromatography with conductivity detection on a Dionex ICS-3000 system (Dionex, http://www.dionex.com) as described in Hubberten et al.Citation7 A clustering of leaf ion content and fresh weight data showed that phosphate treatment clearly separated all samples, indicating the greater impact of phosphate starvation on plant metabolism as compared to the short day treatment (). Remarkably, mycorrhizal colonization of plants grown at short days and at low phosphate shifted wildtype plants toward the +P group. This again highlights the importance of mycorrhizal phosphate uptake for plant growth and metabolism, which results in an increased C/P ratio.
A Principal Component Analysis (PCA) of ion content and fresh weight data was carried out to determine the influence of mycorrhizal colonization and phosphate nutrition on plant growth and metabolism (). PC1 was describing more than 60% of the variance and correlates to C/P ratio of the plants. Plants grown at low phosphate and standard light regime located to one end of this PC axis, and plants grown with moderate phosphate and short day regime represent a low C/P ratio and locate to the other end of this axis. PCA resulted in a clear separation of all 4 experimental conditions, with the exception of the mycorrhizal wildtype plants grown at low phosphate (20 μM). In these plants, mycorrhizal colonization resulted in a clear shift toward a lower C/P ratio due to mycorrhizal phosphate uptake. Under moderate phosphate fertilization (1 mM), mycorrhizal wt plants did weakly separate from the other plants of the corresponding condition, indicating a reduced impact of mycorrhizal phosphate transfer for the plant metabolism and growth under moderately high phosphate nutrition. It is worth mentioning, that mycorrhizal colonization did not lead to a significant growth or metabolic response in mtha1-2 mutants. This underscores first a loss of phosphate transfer due to the non-functional H+-ATPase in mtha1-2 mutants, but also demonstrates that even in the absence of mycorrhizal nutrient uptake, the carbon transfer to AM fungi does not significantly influence plant growth and leaf metabolism under the light and nutrient fertilization regimes applied here.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
This work was supported by the Max-Planck-Society.
References
- Krajinski F, Courty P-E, Sieh D, Franken P, Zhang H, Bucher M, Gerlach N, Kryvoruchko I, Zoeller D, Udvardi M, et al. The H+-ATPase HA1 of Medicago truncatula is essential for phosphate transport and plant growth during arbuscular mycorrhizal symbiosis. Plant Cell 2014; 26:1808-17; PMID:24781114; http://dx.doi.org/10.1105/tpc.113.120436
- Schliemann W, Ammer C, Strack D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008; 69:112-46; PMID:17706732; http://dx.doi.org/10.1016/j.phytochem.2007.06.032
- Schweiger R, Baier MC, Persicke M, Müller C. High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat Commun 2014; 5:3886; PMID:24848943; http://dx.doi.org/10.1038/ncomms4886
- Fester T, Fetzer I, Buchert S, Lucas R, Rillig M, Härtig C. Towards a systemic metabolic signature of the arbuscular mycorrhizal interaction. Oecologia 2011; 167:913-24; PMID:21643790; http://dx.doi.org/10.1007/s00442-011-2037-6
- Sieh D, Watanabe M, Devers EA, Brueckner F, Hoefgen R, Krajinski F. The arbuscular mycorrhizal symbiosis influences sulfur starvation responses of Medicago truncatula. New Phytologist 2013; 197:606-16; PMID:23190168; http://dx.doi.org/10.1111/nph.12034
- Wang E, Yu N, Bano SA, Liu C, Miller AJ, Cousins D, Zhang X, Ratet P, Tadege M, Mysore KS, et al. A H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell 2014; 26:1818-30; PMID:24781115; http://dx.doi.org/10.1105/tpc.113.120527
- Hubberten H-M, Klie S, Caldana C, Degenkolbe T, Willmitzer L, Hoefgen R. Additional role of O-acetylserine as a sulfur status-independent regulator during plant growth. Plant J 2012; 70:666-77; PMID:22243437; http://dx.doi.org/10.1111/j.1365-313X.2012.04905.x
