Abstract
The ILK, PINCH, Parvin (IPP) complex regulates adhesion and migration via binding of ILK to β1 integrin and α−parvin thus linking focal adhesions to actin cytoskeleton. ILK also binds the adaptor protein PINCH which connects signaling proteins including Rsu1 to the complex. A recent study of Rsu1 and PINCH1 in non-transformed MCF10A human mammary epithelial cells revealed that the siRNA-mediated depletion of either Rsu1 or PINCH1 decreased the number of focal adhesions (FAs) and altered the distribution and localization of FA proteins. This correlated with reduced adhesion, failure to spread or migrate in response to EGF and a loss of actin stress fibers and caveolae. The depletion of Rsu1 caused significant reduction in PINCH1 implying that Rsu1 may function in part by regulating levels of PINCH1. However, Rsu1, but not PINCH1, was required for EGF-induced activation of p38 Map kinase and ATF2 phosphorylation, suggesting a Rsu1 function independent from the IPP complex. Reconstitution of Rsu1-depleted cells with a Rsu1 mutant (N92D) that does not bind to PINCH1 failed to restore FAs or migration but did promote IPP-independent spreading and constitutive as well as EGF-induced p38 activation. In this commentary we discuss p38 activity in adhesion and how Rsu1 expression may be linked to Map kinase kinase (MKK) activation and detachment-induced stress kinase signaling.
Abbreviations
| FA | = | focal adhesion |
| FAK | = | focal adhesion kinase |
| ILK | = | Integrin linked kinase |
| IPP | = | Intergrin linked kinase-PINCH-Parvin |
| LRR | = | leucine rich repeat |
| MKK | = | Map kinase kinase |
| NLS | = | nuclear localization sequence |
| RALGEF | = | Ral Guanine nucleotide exchange factor |
| ROS | = | reactive oxygen species |
| Rsu1 | = | Ras suppressor protein 1 |
Introduction
The IPP complex, centrally composed of integrin linked-kinase (ILK) and the adaptor proteins PINCH and parvin, is an organizing and signaling network for integrin adhesion. The composition and the functions of the widely expressed complex have been the subjects of recent comprehensive reviews.Citation1-4 In brief, the complex is organized by PINCH1 binding to ILK with PINCH1 serving as an adaptor binding to Nck2 and Rsu1. ILK links the complex to integrins via binding of its carboxyl terminal domain to β subunits of integrins and associates with the actin cytoskeleton through the binding of α parvin,Citation5 thus linking the complex to integrin activation, actin cytoskeleton remodeling,Citation6,7 and FA formation.Citation3,6 Additionally, a novel LIM only domain protein whose expression is elevated in metastatic tumors has been identified as a binding partner bound to the ILK pseudokinase domain.Citation8
The regulatory function of PINCH in adhesion depends on its association with multiple accessory proteins.Citation7,9,10 PINCH1 and 2 consist of 5 LIM domains and a carboxyl terminal nuclear localization signal (NLS)Citation11,12 and both bind to an ILK ankyrin repeat through their N terminal LIM1 domain.Citation13-15 The IPP complex connects integrins to growth factor signaling through the binding of LIM4 of PINCH1 to Nck2.Citation16,17 PINCH 1 and 2 are highly homologous in LIM domains 1-4 but diverge sufficiently in LIM5 that Rsu1 binds LIM5 of PINCH1 but not PINCH2.Citation18 Because PINCH1 can substitute for PINCH2 in development but the not the reverse, there appears to be an essential role for PINCH1-LIM5 interaction in survival.Citation7,19 In addition to binding Rsu1, the LIM5 domain of PINCH1 has also been reported to bind protein phosphatase-1α (PP1α) as well as Thymosin β4, which maintains pools of actin monomers in cells.Citation20,21
Rsu1 is a leucine rich repeat (LRR) protein that binds to PINCH1 with high affinity via its LRR domainCitation18,22 and co-localizes with PINCH1 at sites of focal adhesions in mammalian cells and muscle cell attachment in Drosophila.Citation18,22-24 The inhibition of PINCH-ILK or PINCH-Rsu1 interaction resulted in decreased cell spreading and reduced motility of mammalian cells.Citation18,25,26 In 2 large scale siRNA-mediated screens Rsu1 and PINCH1 were independently identified as proteins required for migration of the mammary epithelial cells.Citation27,28
Rsu1 contributes to cell adhesion and spreading in part via its role in IPP function. Ectopic expression of Rsu1 in NIH 3T3 mouse fibroblasts increased cell spreading and the accumulation of actin, while shRNA-mediated Rsu1 depletion reduced cell adhesion in mammalian cells and its deletion decreased integrin-dependent functions in Drosophila.Citation18,22,29 The ectopic expression of Rsu1 cDNA also prevented Ras transformation-induced agar growth and inhibited anchorage independent growth of human tumor cell lines.Citation30-32 Rsu1 did not inhibit Ras GTPaseCitation29 but instead altered small G protein signaling downstream of Ras including the inhibition of ROCK, Jun kinase and p38 kinase.Citation18,29,31
Multiple reports correlated changes in the level of Rsu1 with altered Jun kinase activity in Drosophila as well as mammalian cells. Transient expression of Rsu1 in Cos1 cells inhibited growth factor-induced Jun kinase activity,Citation29 and the ectopic expression of Rsu1 reduced Jun kinase phosphorylation and apoptosis in both PINCH1-deleted mouse primitive endoderm cellsCitation24 and ILK-deleted neuroprogenitor stem cells.Citation33 Furthermore, Rsu1 is required for the viability of Drosophila embryos with disrupted PINCH-ILK binding.Citation34 Hence, Rsu1-dependent regulation of stress induced kinase activity suggested that it could link survival signaling to conditions of perturbed adhesion.
Our recent study examining the function of Rsu1 and PINCH1 in mammary epithelial cell adhesion and EGF-induced migration demonstrated a critical role for Rsu1 and the IPP complex in proper organization of FA sites and their link to actin cytoskeleton.Citation35 Additionally, Rsu1 was required for EGF-dependent p38 Map kinase signaling and this function was independent of its interaction with the IPP complex. In this commentary we discuss the potential role(s) for Rsu1 in EGF-induced Map kinase kinase (MKK) activation and phosphorylation of p38 in MCF10A cells.
Rsu1 and PINCH1 Contributions to Focal Adhesions and Caveoli are Required for Migration
Investigation of the requirement for Rsu1-PINCH1 interaction in the regulation of cell adhesion, spreading and migration in MCF10A mammary epithelial cells suggested that both proteins contribute to similar functions. Previous studies established that Rsu1-PINCH1 binding was required for cell adhesion and PINCH1 functionality.Citation3,4,14,18,23,36,37 Our study confirmed that independent depletion of Rsu1 and PINCH1 resulted in decreased cell adhesion, spreading and migration in MCF10A cells.Citation35 This change appeared to be a direct consequence of a disruption in the organization of focal adhesion sites as demonstrated by the altered distribution and localization of the FA proteins paxillin, vinculin, talin, and β1 integrin as well as a reduction in the levels of phosphorylated FAK (Tyr 397). Most phenotypes associated with decreased levels of either Rsu1 or PINCH1 were more severe in the PINCH1-depleted cells. As observed previously, the depletion of Rsu1 caused significant reduction in PINCH1, while PINCH1 depletion resulted in only a modest reduction in Rsu1, implying that Rsu1 functions in the regulation of PINCH1 stabilization and that the phenotype observed in Rsu1-depleted cells may be partially due to decreased PINCH1 levels.Citation18,22,34
While ILK expression was slightly affected by Rsu1 reduction, PINCH1 depletion significantly decreased ILK levels. Phenotypes previously observed in ILK deficient cells were noted in Rsu1- and PINCH1-depleted cells including the release of caveolin from the FAsCitation38-40 and the localization and distribution of β1 integrin in endosome like structures. These changes were primarily linked to loss of PINCH1-ILK function. Similarly, Rsu1- and PINCH1-depleted cells displayed a loss of actin stress fibers. However, PINCH1 depletion also caused increased phosphorylation of actin regulatory proteins VASP and cofilin. The loss of actin stress fibers was likely the outcome of defective ILK-parvin association resulting from fewer ILK molecules available for parvin binding, which is critical for actin mediated functions in adhesion.Citation4,41
While most of the phenotypes exhibited by Rsu1-depleted cells were not as severe as those lacking PINCH1, cell spreading was equally affected by Rsu1 or PINCH1 depletion. Rac activity has been linked to spreading, and both Rsu1 and PINCH1 depleted cells exhibited decreased constitutive Rac1 activity. However, EGF stimulation restored Rac-GTP regardless of the level of Rsu1 or PINCH1 and a recent report demonstrated that Rac is not required for spreading of fibroblasts,Citation42 hence, it is unlikely that the Rac defect alone impaired spreading in Rsu1 or PINCH1 deficient cells. Reconstitution of Rsu1-depleted cells with a Rsu1 mutant (N92D) that fails to bind PINCH1 restored spreading in MCF10A cells otherwise defective due to the depletion of the endogenous Rsu1, thus implicating a Rsu1-dependent event controlling spreading. ILK, but not Rac, is required for lumen formation by mammary epithelial cells.Citation43 The formation of acini with luminal clearance by N92D-Rsu1 cells supported the idea that the inhibition of Rac activation resulting from absence of Rsu1-PINCH1 interaction in MCF10A cells was not critical.
Expression of the N92D-Rsu1 protein in Rsu1-depleted cells resulted in small FAs compared to wt-Rsu1, and only wt-Rsu1 restored migration, indicating that binding of Rsu1 to PINCH1-ILK-parvin is required for the proper regulation of adhesion and migration. Because the size of FAs correlates with migratory activity,Citation44 the reduced size of FA sites in cells expressing only N92D-Rsu1 likely contributed to reduced migration. In support of this analysis, Rsu1-PINCH1-ILK interaction is required for rescue of muscle cell attachment migration defects in a myosin phosphatase hypercontraction mutant of Drosophila.Citation45
Rsu1 is Required for EGF-induced Activation of the p38 Map Kinase Signaling
Previous work demonstrated that modulation of Rsu1 levels altered ROCK,Citation31,46 Jun kinase and p38 Map kinase activity.Citation18,22,29 The exact mechanism by which this occurred, and whether it resulted from alterations in IPP functions, is not entirely clear. It is worth noting that while Rsu1 expression blocked Ras-dependent transformation of NIH3T3 fibroblasts as measured by agar growth, Rsu1 expression did not block v-src-induced transformation of the same cells.Citation30 The differences between v-src and Ras transformation include the mechanisms by which they activate PI-3-kinase signalingCitation47,48 and the Ras-dependent increase in Jun kinase which is required for Ras transformation.Citation49,50 In addition, ROS elevation leading to p38 activation and RALGEF stimulation are unique changes that occur in Ras-transformed but not src-transformed cells.Citation51-53 Hence, the reduction in Jun kinase and the increase in p38 signaling due to Rsu1 expression may be mechanistically linked to the Rsu1 specificity for inhibition of Ras transformation.
p38 Map kinase signaling is required for cell spreading and migration in response to diverse signalsCitation54-57 and EGF-dependent MCF10A cell migration was blocked by inhibitors of p38 Map kinase. The depletion of Rsu1, but not PINCH1, blocked EGF- induced p38 phosphorylation in MCF10A cells suggesting a unique function for Rsu1 in activation of this pathway. The wt-Rsu1, and also the N92D-Rsu1 mutant, restored EGF-induced p38 activity in Rsu1 depleted cells supporting the finding of an Rsu1 function independent of IPP signaling. Furthermore, the constitutive and inducible p38 phosphorylation and the recovery of cell spreading resulting from expression of the N92D-Rsu1 mutant suggested that Rsu1 contributes to cell spreading by promoting signaling through the p38 Map kinase. Data from several studies support a role for p38 in actin cytoskeletal regulation and spreading. For example, WAVE3 promotes cell motility via the p38 Map kinase pathway and MMP expressionCitation58 and signaling through p38 is required for actin polymerization and cell migration in smooth muscle cellsCitation56 and intestinal epithelial cells.Citation57 The treatment of oligodendrocytes with thymosin β4, a PINCH1 binding and actin regulatory protein, also increased p38 Map kinase activityCitation59 and ILK dependent activation of p38 regulated actin polymerization in vascular smooth muscle cells.Citation60 While these studies did not probe for a link to Rsu1 it may have been a contributing factor to the alteration in p38 Map kinase.
p38 Map kinase is an important regulator of cell death and survival under conditions of stress, including detachment-induced stress that occurs during acini formation in MCF10A cells. In this process activated p38 is thought to be essential for apoptotic signals required for luminal clearance.Citation61 In cells depleted of Rsu1 the defect in p38 activation was restricted to an EGF-induced activation pathway. Hence, the detachment of Rsu1-depleted MCF10A cells resulted in p38 phosphorylation and allowed formation of acini exhibiting luminal clearance.
There are a number of ways in which p38 signaling participates in essential cellular processes. Activation of p38 is detected in spreading and migration as noted above. Also, phosphorylated p38 can function to activate checkpoint controls that allow cell survival under conditions of stress in MCF10A cells.Citation62 p38 and its target ATF2 were required for survival in embryonic liver Citation63 and some leukemic cells.Citation64 Several lines of evidence also support a survival function for Rsu1. In Drosophila embryos with disruption of PINCH-ILK binding the depletion of Rsu1 resulted in lethality independent of PINCH1 localization or stabilization.Citation34 The depletion of PINCH1 in mouse primitive endoderm cells resulted in a decrease of Rsu1 concomitant with increased Jun kinase and Bax activation and reduced Bcl-2 levels, and the introduction of Rsu1 decreased Jun kinase activity.Citation24 It is not clear if Rsu1 functions to regulate p38 in these systems but it could contribute to survival by activating a p38 checkpoint control. The elevated levels of p38 activation displayed by the Rsu1 mutant and the lack of p38 activity exhibited by the Rsu1-depleted cells supports a function for Rsu1 in promoting cell survival through a p38 mediated mechanism. In addition, this provides an explanation for the inability of Rsu1 depleted MC10A cells to survive indefinitely.
There are several relevant control points for p38 phosphorylation including its activation by MKK4 or MKK3 and MKK6. In MCF10A cells, detachment-induced p38 activation occurs via phosphorylation primarily by MKK6Citation61 but this pathway is poorly activated by EGF stimulation in these cells (). Hence, the less adherent N92D-Rsu1 expressing cells, which exhibit constitutive p38 phosphorylation, might be expected to have MKK6 activation as a result of decreased FAs and FA function. In 293T cells, which can survive without strong adhesion to substrate, the disruption of Rsu1-PINCH1 association led to reduced adhesion and enhanced p38 and Jun kinase activation.Citation18
Figure 1. siRNA-mediated depletion of Rsu1 blocks activation of MKK4 in MCF10A cells. MCF10A cells transfected with a Rsu1 specific or a negative control siRNA were stimulated with EGF (10ng/ml) at 96 hours post transfection and lysates were harvested and examined by western blotting as described previously.Citation35 Antibodies for the expression of proteins include: phospho-MKK4 Ser257 (Cell Signaling Technology #4514), MKK4 (Santa Cruz Biotechnology #166168), phospho-MKK3 Ser189/MKK6 Ser207 (Cell Signaling Technology #9231) Tubulin (Santa Cruz Biotechnology #8035) was used as loading control.
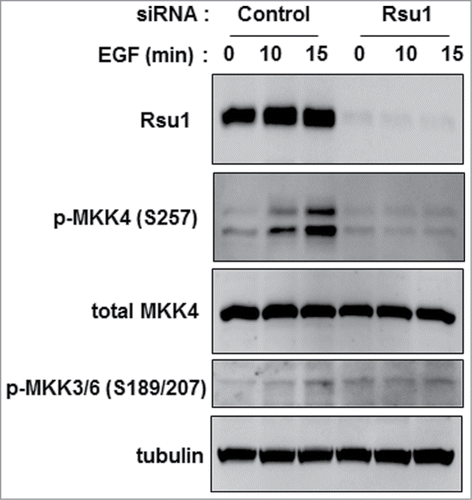
However, reconstitution of Rsu1 depleted MCF10A cells with N92D-Rsu1 allowed p38 phosphorylation by EGF suggesting that another pathway, likely MKK4, is activated by EGF. The results in clearly demonstrate that siRNA-mediated depletion of Rsu1 blocked the stimulation of MKK4 phosphorylation in response to EGF in MCF10A cells. Hence, this indicates that the loss of Rsu1 has an impact on p38 Map kinase signaling via the inhibition of MKK4 activation. Because MKK4 can activate both p38 and Jun kinase depending on cell specific stimulatory and regulatory context, it may be the critical node for Rsu1 regulation of these kinases ().
Figure 2. The absence of Rsu1-PINCH1 binding alters MKK4 and p38 Map kinase activation in MCF10A cells. (Left) Expression of either endogenous Rsu1 protein or wt-Rsu1 in the absence of endogenous protein allows activation of MKK4 and phosphorylation of p38 Map kinase in MCF10A cells. (Middle) In cells that are detached from substrate, or depleted of Rsu1, integrin engagement and focal adhesion formation are reduced or absent. This results in a block in the activation of MKK4, but MCF10A cell detachment leads to the activation of p38 by MKK6. (Right) The reconstitution of Rsu1-depleted cells with N92D-Rsu1 does not completely restore FA formation but it does promote MKK4 activation.
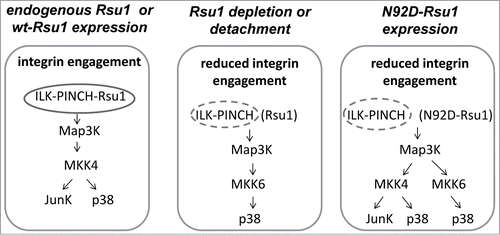
MKK4 is well characterized as tumor suppressor in many solid tumors including breast, ovarian and liver. In addition to MKK4 deletion or mutation in tumors, the loss of Rsu1 activity may interfere with MKK4 signaling and thereby contribute to the loss of tumor suppressor activity. Genomic deletions in Rsu1 have been detected in both a subset of human hepatocellular carcinomas as well as in gliomasCitation65,66, and according to recent reports the depletion of Rsu1 in prostate tumors occurs via stroma-produced miRs.Citation67,68 Hence, the connection of Rsu1 to MKK4, p38, Jun kinase and ATF2 may be a potential regulatory event in tumorigenesis.
Conclusions
Our recent report identified a defect in the EGF-induced activation of the p38 Map kinase in MCF10A cells depleted of Rsu1. The reconstitution of MCF10A with wt-Rsu1 restored p38 Map kinase activity, FA formation, spreading and migration. In contrast, a mutant of Rsu1 that fails to bind to PINCH1 and the IPP complex restored only responsiveness to EGF-induced action of p38 but not complete formation of FAs or functions dependent on intact FAs. However, the restoration of p38 signaling coincided with functional cell spreading. Collectively, these results confirm a critical role for Rsu1 and IPP complex interaction in proper formation of FA sites and actin cytoskeleton remodeling. More importantly our findings revealed a unique role for Rsu1 in cell spreading and p38 activation that is independent from IPP signaling. Modulation of signaling at the level of MKK4, a common Jun kinase and p38 Map kinase activator, could account for the divergent results obtained following Rsu1 depletion or ectopic expression. Data from several sources suggest that MKK4 may be a critical molecule in this pathway and Rsu1 depletion blocks MKK4 activation by EGF in MCF10A cells. Hence, our current efforts are directed at understanding how Rsu1 differentially regulates p38 and Jun kinase activity and how this regulation is related to adhesion and survival.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr. Bethanie Morrison for insightful comments. The opinions expressed here are those of the authors and should not be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
Funding
The following funding agencies provided support: Congressionally Directed Medical Research Program grant W81XWH-09-2-0056 from the Wound Healing Program (to MLC), the Murtha Cancer Center at Walter Reed National Military Medical Center through Uniformed Services University (to MLC) and W81XWH-10-1-0024 from the Congressionally Directed Medical Research Breast Cancer Program (predoctoral fellowship to RG-N).
References
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer 2010; 10:858-70; PMID:21102636; http://dx.doi.org/10.1038/nrc2967
- Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol 2012; 24:607-13; PMID:22763012; http://dx.doi.org/10.1016/j.ceb.2012.06.003
- Stanchi F, Grashoff C, Nguemeni Yonga CF, Grall D, Fassler R, Van Obberghen-Schilling E. Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci 2009; 122:1800-11. http://jcs.biologists.org/content/122/11/1800.full.pdf; http://dx.doi.org/10.1242/jcs.044602
- Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 2010; 29:281-91. http://www.nature.com/emboj /journal /v29/n2/pdf/emboj2009376a.pdf; http://dx.doi.org/10.1038/emboj.2009.376
- Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 2001; 153:585-98. http://pubmedcentralcanada.ca/picrender.cgi?accid=PMC2190577&blobtype=pdf; http://dx.doi.org/10.1083/jcb.153.3.585
- Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev 2003; 17:926-40. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC196029/pdf/08X.pdf; http://dx.doi.org/10.1101/gad.255603
- Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci 2005; 118:2913-21. http://jcs.biologists.org/content/118/13/2913.full.pdf; http://dx.doi.org/10.1242/jcs.02422
- Peng H, Talebzadeh-Farrooji M, Osborne MJ, Prokop JW, McDonald PC, Karar J, Hou Z, He M, Kebebew E, Orntoft T, et al. LIMD2 is a small LIM-only protein overexpressed in metastatic lesions that regulates cell motility and tumor progression by directly binding to and activating the integrin-linked kinase. Cancer Res 2014; 74:1390-403; PMID:24590809; http://dx.doi.org/10.1158/0008-5472.CAN-13-1275
- Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr., Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol 2005; 25:3056-62; PMID:15798193; http://dx.doi.org/10.1128/MCB.25.8.3056-3062.2005
- Xu Z, Fukuda T, Li Y, Zha X, Qin J, Wu C. Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem 2005; 280:27631-7. http://www.jbc.org/content/280/30/27631.full.pdf; http://dx.doi.org/10.1074/jbc.M504189200
- Hobert O, Moerman DG, Clark KA, Beckerle MC, Ruvkun G. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J Cell Biol 1999; 144:45-57. http://pubmedcentralcanada.ca/picrender.cgi?accid=PMC2148118&blobtype=pdf; http://dx.doi.org/10.1083/jcb.144.1.45
- Braun A, Bordoy R, Stanchi F, Moser M, Kostka GG, Ehler E, Brandau O, Fassler R. PINCH2 is a new five LIM domain protein, homologous to PINCHand localized to focal adhesions. Exp Cell Res 2003; 284:239-50. http://www.sciencedirect.com/science/article/pii/S0014482702000393; http://dx.doi.org/10.1016/S0014-4827(02)00039-3
- Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci 1999; 112 (Pt 24):4589-99; PMID:10574708
- Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 2002; 115:4777-86. http://jcs.biologists.org/content/115/24/4777.full.pdf; http://dx.doi.org/10.1242/jcs.00166
- Zhang Y, Chen K, Guo L, Wu C. Characterization of PINCH-2, a new focal adhesion protein that regulates the PINCH-1-ILK interaction, cell spreading, and migration. J Biol Chem 2002; 277:38328-38; PMID:12167643; http://dx.doi.org/10.1074/jbc.M205576200
- Velyvis A, Vaynberg J, Yang Y, Vinogradova O, Zhang Y, Wu C, Qin J. Structural and functional insights into PINCH LIM4 domain-mediated integrin signaling. Nat Struct Biol 2003; 10:558-64; PMID:12794636; http://dx.doi.org/10.1038/nsb938
- Tu Y, Li F, Wu C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell 1998; 9:3367-82. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC25640/pdf/mk003367.pdf; http://dx.doi.org/10.1091/mbc.9.12.3367
- Dougherty GW, Chopp T, Qi SM, Cutler ML. The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp Cell Res 2005; 306:168-79. http://www.sciencedirect.com/science/article/pii/S0014482705000418; http://dx.doi.org/10.1016/j.yexcr.2005.01.025
- Stanchi F, Bordoy R, Kudlacek O, Braun A, Pfeifer A, Moser M, Fassler R. Consequences of loss of PINCH2 expression in mice. J Cell Sci 2005; 118:5899-910; PMID:16317048; http://dx.doi.org/10.1242/jcs.02686
- Eke I, Koch U, Hehlgans S, Sandfort V, Stanchi F, Zips D, Baumann M, Shevchenko A, Pilarsky C, Haase M, et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J Clin Invest 2010; 120:2516-27; PMID:20530873; http://dx.doi.org/10.1172/JCI41078
- Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004; 432:466-72; PMID:15565145; http://dx.doi.org/10.1038/nature03000
- Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JR, 3rd, Beckerle MC. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol 2004; 167:1019-24; PMID:15596544; http://dx.doi.org/10.1083/jcb.200408090
- Dougherty GW, Jose C, Gimona M, Cutler ML. The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. Eur J Cell Biol 2008; 87:721-34. http://www.sciencedirect.com/science/article/pii/S0171933508000502 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2600675/pdf/nihms67776.pdf; http://dx.doi.org/10.1016/j.ejcb.2008.02.011
- Montanez E, Karakose E, Tischner D, Villunger A, Fassler R. PINCH-1 promotes Bcl-2-dependent survival signalling and inhibits JNK-mediated apoptosis in the primitive endoderm. J Cell Sci 2012; 125:5233-40; PMID:22946061; http://dx.doi.org/10.1242/jcs.112029
- Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 1999; 19:2425-34. http://pubmedcentralcanada.ca/picrender.cgi?accid=PMC84035&blobtype=pdf.
- Zhang Y, Guo L, Chen K, Wu C. A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem 2002; 277:318-26. http://www.jbc.org/content/277/1/318.full.pdf; http://dx.doi.org/10.1074/jbc.M108257200
- Winograd-Katz SE, Itzkovitz S, Kam Z, Geiger B. Multiparametric analysis of focal adhesion formation by RNAi-mediated gene knockdown. J Cell Biol 2009; 186:423-36; PMID:19667130; http://dx.doi.org/10.1083/jcb.200901105
- Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol 2008; 10:1027-38; PMID:19160483; http://dx.doi.org/10.1038/ncb1762
- Masuelli L, Cutler ML. Increased expression of the Ras suppressor Rsu-1 enhances Erk-2 activation and inhibits Jun kinase activation. Mol Cell Biol 1996; 16:5466-76. http://pubmedcentralcanada.ca/picrender.cgi?accid=PMC231547&blobtype=pdf.
- Cutler ML, Bassin RH, Zanoni L, Talbot N. Isolation of rsp-1, a novel cDNA capable of suppressing v-Ras transformation. Mol Cell Biol 1992; 12:3750-6. http://pubmedcentralcanada.ca/picrender.cgi?accid=PMC360236&blobtype=pdf.
- Vasaturo F, Dougherty GW, Cutler ML. Ectopic expression of Rsu-1 results in elevation of p21CIP and inhibits anchorage-independent growth of MCF7 breast cancer cells. Breast Cancer Res Treat 2000; 61:69-78; PMID:10930091; http://dx.doi.org/10.1023/A:1006462323260
- Tsuda T, Marinetti MR, Masuelli L, Cutler ML. The Ras suppressor RSU-1 localizes to 10p13 and its expression in the U251 glioblastoma cell line correlates with a decrease in growth rate and tumorigenic potential. Oncogene 1995; 11:397-403; PMID:7624154
- Porcheri C, Suter U, Jessberger S. Dissecting integrin-dependent regulation of neural stem cell proliferation in the adult brain. J Neurosci 2014; 34:5222-32; PMID:24719101; http://dx.doi.org/10.1523/JNEUROSCI.4928-13.2014
- Elias MC, Pronovost SM, Cahill KJ, Beckerle MC, Kadrmas JL. A crucial role for Ras suppressor-1 (RSU-1) revealed when PINCH and ILK binding is disrupted. J Cell Sci 2012; 125:3185-94; PMID:22467865; http://dx.doi.org/10.1242/jcs.101386
- Gonzalez-Nieves R, Desantis AI, Cutler ML. Rsu1 contributes to regulation of cell adhesion and spreading by PINCH1-dependent and - independent mechanisms. J Cell Commun Signal 2013; 7:279-93; PMID:23765260; http://dx.doi.org/10.1007/s12079-013-0207-5
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 2006; 7:20-31. http://www.nature.com/nrm/journal/v7/n1/pdf/nrm1789.pdf; http://dx.doi.org/10.1038/nrm1789
- Zervas CG, Psarra E, Williams V, Solomon E, Vakaloglou KM, Brown NH. A central multifunctional role of integrin-linked kinase at muscle attachment sites. J Cell Sci 2011; 124:1316-27; PMID:21444757; http://dx.doi.org/10.1242/jcs.081422
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 2009; 10:843-53; PMID:19904298; http://dx.doi.org/10.1038/nrm2799
- Wickstrom SA, Lange A, Hess MW, Polleux J, Spatz JP, Kruger M, Pfaller K, Lambacher A, Bloch W, Mann M, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 2010; 19:574-88; PMID:20951348; http://dx.doi.org/10.1016/j.devcel.2010.09.007
- Meyer A, van Golen CM, Boyanapalli M, Kim B, Soules ME, Feldman EL. Integrin-linked kinase complexes with caveolin-1 in human neuroblastoma cells. Biochemistry 2005; 44:932-8. http://pubs.acs.org/doi/abs/10.1021/bi048619r; http://dx.doi.org/10.1021/bi048619r
- Fukuda K, Gupta S, Chen K, Wu C, Qin J. The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell 2009; 36:819-30. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2796127/pdf/nihms162425.pdf; http://dx.doi.org/10.1016/j.molcel.2009.11.028
- Steffen A, Ladwein M, Dimchev GA, Hein A, Schwenkmezger L, Arens S, Ladwein KI, Margit Holleboom J, Schur F, Victor Small J, et al. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci 2013; 126:4572-88; PMID:23902686; http://dx.doi.org/10.1242/jcs.118232
- Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol 2013; 15:17-27; PMID:23263281; http://dx.doi.org/10.1038/ncb2646
- Kim DH, Wirtz D. Focal adhesion size uniquely predicts cell migration. FASEB J 2013; 27:1351-61; PMID:23254340; http://dx.doi.org/10.1096/fj.12-220160
- Pronovost SM, Beckerle MC, Kadrmas JL. Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Drosophila melanogaster Muscle Hypercontraction Mutants. PLoS Genet 2013; 9:e1003406; PMID:23555310; http://dx.doi.org/10.1371/journal.pgen.1003406
- Donthamsetty S, Bhave VS, Mars WM, Bowen WC, Orr A, Haynes MM, Wu C, Michalopoulos GK. Role of PINCH and Its Partner Tumor Suppressor Rsu-1 in Regulating Liver Size and Tumorigenesis. PLoS One 2013; 8:e74625; PMID:24058607; http://dx.doi.org/10.1371/journal.pone.0074625
- Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, Mills GB. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem 2001; 276:27455-61; PMID:11337495; http://dx.doi.org/10.1074/jbc.M100556200
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 1994; 370:527-32; PMID:8052307; http://dx.doi.org/10.1038/370527a0
- Cellurale C, Sabio G, Kennedy NJ, Das M, Barlow M, Sandy P, Jacks T, Davis RJ. Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol Cell Biol 2011; 31:1565-76; PMID:21282468; http://dx.doi.org/10.1128/MCB.01122-10
- Nielsen C, Thastrup J, Bottzauw T, Jaattela M, Kallunki T. c-Jun NH2-terminal kinase 2 is required for Ras transformation independently of activator protein 1. Cancer Res 2007; 67:178-85; PMID:17210697; http://dx.doi.org/10.1158/0008-5472.CAN-06-2801
- Deichman GI, Kashkina LM, Mizenina OA, Gorojanskaya EG, Nikiforov MA, Gudkov AV, Dyakova NA, Komelkov AV, Prilutskaya MO, Kushlinsky NE, et al. Mechanisms of unusually high antioxidant activity of RSV-SR-transformed cells and of its suppression by activated p21ras. Int J Cancer 1996; 66:747-52; PMID:8647644; http://dx.doi.org/10.1002/(SICI)1097-0215(19960611)66:6%3c747::AID-IJC7%3e3.0.CO;2-
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997; 275:1649-52; PMID:9054359; http://dx.doi.org/10.1126/science.275.5306.1649
- Maslikowski BM, Neel BD, Wu Y, Wang L, Rodrigues NA, Gillet G, Bedard PA. Cellular processes of v-Src transformation revealed by gene profiling of primary cells–implications for human cancer. BMC Cancer 2010; 10:41; PMID:20152043; http://dx.doi.org/10.1186/1471-2407-10-41
- Dobreva I, Waeber G, James RW, Widmann C. Interleukin-8 secretion by fibroblasts induced by low density lipoproteins is p38 MAPK-dependent and leads to cell spreading and wound closure. J Biol Chem 2006; 281:199-205; PMID:16251188; http://dx.doi.org/10.1074/jbc.M508857200
- Varon C, Rottiers P, Ezan J, Reuzeau E, Basoni C, Kramer I, Genot E. TGFbeta1 regulates endothelial cell spreading and hypertrophy through a Rac-p38-mediated pathway. Biol Cell 2008; 100:537-50; PMID:18387002; http://dx.doi.org/10.1042/BC20080021
- Pichon S, Bryckaert M, Berrou E. Control of actin dynamics by p38 MAP kinase - Hsp27 distribution in the lamellipodium of smooth muscle cells. J Cell Sci 2004; 117:2569-77; PMID:15128872; http://dx.doi.org/10.1242/jcs.01110
- Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 2004; 279:44513-21; PMID:15316018; http://dx.doi.org/10.1074/jbc.M406253200
- Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 2005; 308:135-45; PMID:15907837; http://dx.doi.org/10.1016/j.yexcr.2005.04.011
- Santra M, Chopp M, Zhang ZG, Lu M, Santra S, Nalani A, Morris DC. Thymosin beta 4 mediates oligodendrocyte differentiation by upregulating p38 MAPK. Glia 2012; 60:1826-38; PMID:23073962; http://dx.doi.org/10.1002/glia.22400
- Esfandiarei M, Yazdi SA, Gray V, Dedhar S, van Breemen C. Integrin-linked kinase functions as a downstream signal of platelet-derived growth factor to regulate actin polymerization and vascular smooth muscle cell migration. BMC Cell Biol 2010; 11:16; PMID:20178627; http://dx.doi.org/10.1186/1471-2121-11-16
- Wen HC, Avivar-Valderas A, Sosa MS, Girnius N, Farias EF, Davis RJ, Aguirre-Ghiso JA. p38alpha Signaling Induces Anoikis and Lumen Formation During Mammary Morphogenesis. Sci Signal 2011; 4:ra34; PMID:21610252; http://dx.doi.org/10.1126/scisignal.2001684
- Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ, Jr. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 2001; 411:102-7; PMID:11333986; http://dx.doi.org/10.1038/35075107
- Breitwieser W, Lyons S, Flenniken AM, Ashton G, Bruder G, Willington M, Lacaud G, Kouskoff V, Jones N. Feedback regulation of p38 activity via ATF2 is essential for survival of embryonic liver cells. Genes Dev 2007; 21:2069-82; PMID:17699753; http://dx.doi.org/10.1101/gad.430207
- Ringshausen I, Dechow T, Schneller F, Weick K, Oelsner M, Peschel C, Decker T. Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia 2004; 18:1964-70; PMID:15483673; http://dx.doi.org/10.1038/sj.leu.2403544
- Wu HT, Hajirasouliha I, Raphael BJ. Detecting independent and recurrent copy number aberrations using interval graphs. Bioinformatics 2014; 30:i195-i203; PMID:24931984; http://dx.doi.org/10.1093/bioinformatics/btu276
- Nalesnik MA, Tseng G, Ding Y, Xiang GS, Zheng ZL, Yu Y, Marsh JW, Michalopoulos GK, Luo JH. Gene deletions and amplifications in human hepatocellular carcinomas: correlation with hepatocyte growth regulation. Am J Pathol 2012; 180:1495-508; PMID:22326833; http://dx.doi.org/10.1016/j.ajpath.2011.12.021
- Josson S, Gururajan M, Hu P, Shao C, Chu GC, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al. miR-409-3p/-5p Promotes Tumorigenesis, Epithelial-to-Mesenchymal Transition, and Bone Metastasis of Human Prostate Cancer. Clin Cancer Res 2014; 20:4636-46; PMID:24963047; http://dx.doi.org/10.1158/1078-0432.CCR-14-0305
- Josson S, Gururajan M, Sung SY, Hu P, Shao C, Zhau HE, Liu C, Lichterman J, Duan P, Li Q, et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene 2014; Advance online publication, 28 July 2014; PMID:25065597; http://dx.doi.org/10.1038/onc.2014.212
