Abstract
Myoblast transplantation (MT) is a method to introduce healthy genes into abnormal skeletal muscle. It has been considered as a therapeutic modality in the last few decades for diseases such as Duchenne Muscular Dystrophy (DMD). However, challenges including cell death and poor graft engraftment have limited its application. The current experiment utilizes MMP1 gene transfer to improve the efficacy of myoblast transplantation into the diseased dystrophic skeletal muscle of mdx mice. Our results indicated that MMP1 expression can promote myogenic differentiation and fusion capacities, increase migration of MMP1 expressing myoblasts in vitro, as well as improve engraftment of dystrophin positive myofibers in vivo. Taken together, our observation suggests that the addition of MMP1 can overcome limitations in MT and improve its clinical efficacy.
Introduction
Myogenic cell (i.e., muscle derived stem cells [MDSCs], satellite cells or myoblasts) transplantation is one of the best approaches to introducing the normal dystrophin gene into the diseased muscle of Duchenne Muscular Dystrophy (DMD) and other muscular dystrophic diseases.Citation1-5 However, multiple injections are often required to achieve sufficient efficacy due to the poor survival rate and migration ability of the transplanted myogenic cells.Citation1,6 Developing techniques that can promote the distribution of the introduced myogenic cells and/or the dystrophin gene within dystrophic muscle would be of significant clinical value. The secondary pathological process that poses a considerable problem for DMD patients includes fibrous scar tissue formation; patients’ muscles are often replaced by fibrous scar tissue (and thus become very weak) during their adolescence.Citation7,8 Fibrous tissue is one of the major obstacles that impedes the muscle healing process as it forms a physical barrier that limits muscle cell migration and fusion, which in turn creates an unsuitable environment for regenerating myofibers and delays muscle healing.Citation9,10 Thus, therapeutic interventions that can reverse fibrous scar tissue formation also can facilitate the healing process.
Extracellular matrix (ECM) is the major part of natural muscle architecture that can support muscle functions, such as strength and contraction. However, ECM responds very quickly to injuries for tissues’ prevention, the process also can be promoted to accelerate fibrous scar tissue formation at injured or diseased skeletal muscles. Our previous studies have demonstrated that myogenic cells, including MDSCs, can be induced differentiation into fibrotic cells and transforming growth factor (TGF)-β1 is a key stimulator within the traumatic injured skeletal muscle, indicating the essential role of the environmental influence during muscle tissue healing.Citation11,12 We believe that myoblast transplantation into dystrophic muscle fails not only because of donors’ death and non-engraftment, but also because of the fibrous scar tissue and the fibrotic environment that impede muscle regeneration. Thus, development of a novel therapeutic approach to increase muscle cell migration, remove existing fibrous scar tissue, remodel the ECM of injured and diseased skeletal muscle, and improve muscle regeneration would be very significant.
Matrix metalloproteinase type 1 (MMP1), a naturally occurring collagen-digesting enzyme, which specifically digest collagens type I&III, can eliminate the existing fibrotic scarring in different tissues including skeletal muscle.Citation13-15 Our previous study has shown that MMP1 could improve muscle healing by reversing fibrotic scar tissue formation and enhancing muscle regeneration in traumatically injured skeletal muscle.Citation16,17 MMP1 injection at the injury site can enhance myoblast integration into the dystrophic skeletal muscle of mdx mice, a dystrophic skeletal muscle murine model, by increasing both myogenic cell migration and differentiation capacities.Citation18 MMP1 treatment has also been demonstrated to be beneficial for muscle satellite cell and stem cell function.Citation19 However, the short biological half-life of MMP1 may limit its efficacy in promoting muscle tissue healing and reducing fibrotic tissue formation.Citation20 Therefore, developing therapeutic strategies that can prolong the availability of functional MMP1 at the injury site is of great research interest. The current experiment examined if MMP1 gene therapy could prolong MMP1 availability, prevent fibrosis formation, and increase myogenic cell migration through either local injection or systemic delivery, thereby improving myogenic cell transplantation efficacy in dystrophic skeletal muscle of mdx mice model.
Materials and Methods
Cell growth curve
C2C12 myoblasts were purchased from ATCC, and muscle derived stem cells (MDSCs) were isolated from adult C57BL6J mice (female, 4–6 weeks of age, Jackson lab) Citation21 in our lab. Cells were plated at a density of 5000 cells/well in collagen-coated 6-well plates and cultured in a complete growth medium containing Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere for 120 hours (hrs). At every 24hrs cells were harvested and counted. The approximate Population Doubling Time (PDT) was calculated as: PDT = T ln2/ln(Xe/Xb); where T = incubation time in any units; Xb = cell number at the beginning of the incubation time; Xe = cell number at the end of the incubation time.
MMP1 plasmid constructs
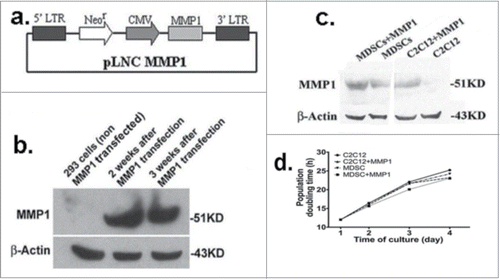
A retrovirus vector, pLNCX2 (Retroviral Vector, CLONETECH) was selected to encode human MMP1 gene. The Bgl II (blunt ended)/Sal I fragment of pCllase I (ATCC, Rockville, MD), including all coding regions of the full-length human MMP1 gene, was cloned into the Not I (blunt ended)/Xho I site of pLNCX2 to generate pLNC MMP1 (). Phoenix 293 cells were transfected (ATCC, Rockville, MD) with pLNC MMP1 using the liposome technique (DOTAP, Boehringer Mannheim), and G418 (500 μg/mL, Sigma-Aldrich, MO) medium was used to select for the transfected cells.
Figure 1. MMP1 plasmid constructions. A retrovirus vector, pLNCX2 (Retroviral Vector, CLONETECH) was selected to encode all coding regions of the full-length human MMP1 gene (A). The package cells of Phoenix 293 were transfected with pLNC MMP1; Western blot analysis indicated the clone cells highly express MMP1 (B). The successful MMP1 gene transfection into C2C12 myoblasts and MDSCs were confirmed by western blot (C). MMP1 gene transfer does not affect the proliferation of C2C12 myoblasts nor MuSCs. No significant different was detected between the MMP1 gene transfected cells and the control cells (D).
Scratch wound migration assaysCitation18
Twelve-well plates were either uncoated or coated with type I collagen or fibronectin. The MMP1 genetically engineered myoblasts and control C2C12 myoblasts (ATCC, Rockville, MD) were cultured in a complete growth medium at 37°C in a 5% CO2 atmosphere until 70% confluency. Artificial wound was created by disrupting the monolayer with a sterile plastic pipette tips. Cells were incubated for 1, 4, 6, and 12 hrs to allow for migration back into the wound area. Cells were then fixed in cold methanol, washed with phosphate-buffered saline (PBS), and then stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) to help visualize cell migration. Northern Eclipse software (Empix Imaging Inc., Mississauga, Canada) was used to quantify the average migration distance of C2C12 myoblasts that traveled past the original wound demarcation.
Single cell migration assayCitation19
We selected a life-cell-image system (Olympus, Precision Plastics) to track genetic engineered C2C12 myoblasts and control C2C12 myoblast cells. Proper environmental conditions were maintained in a microincubator (37°C, 5% CO2), and series of images were analyzed using the NIH ImageJ analysis software to track the centroid positions (x,y) of each cell nuclei. Migration paths were plotted and analyzed by the Chemotaxis and Migration Tool v2.0 from Ibidi. The migration paths of 16 individual cells in different experimental groups were captured and measured, the migration speed and distance were calculated.
Differentiation assays
MMP1 transfected C2C12 myoblasts, MDSCs and their control non-gene-transferred cells were separately plated into 12-well-plates (1 × 105 cell per well) and cultured with differentiation medium (DMEM, 2% horse serum, 1% penicillin/streptomycin) for up to 5 days before fixation (cold acetone for 3 minutes). The myotubes were then stained with 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI) for nuclei specific visualization after 1, 3 and 5 days of culture. Nuclear number over 2 is considered as myotubes and counted. Myotubes were recorded by fluorescent microscopy (Nikon microscope, Nikon, Melville, New York) and calculated. The total number of nuclei and myotubes was counted in 8 random fields (>600 total nuclei) per condition. The fusion index (i.e., the ratio of the nuclei number in myocytes with 2 or more nuclei (myotubes) vs. the total number of nuclei of per random field) was calculated at each time point.
Quantitative real time PCR (qRT-PCR)
C2C12 myoblasts and C2C12-MMP1 transfected myoblasts were plated onto collagen-coated 12-well plates with a density of 2 × 104 cells/well. For the proliferation assay cells were cultured in DMEM proliferation medium; while for the differentiation assay, cells were cultured in muscle differentiation medium (see above). Samples were harvested at 3, 6, 12, 24, 48, and 96 hrs for proliferation assay, and on 1, 3, 5 and 7 day for differentiation assay. Total RNA was isolated by using the RNeasy Plus Mini Kit (Quiagen, USA), and cDNA was synthesized from 1 μg of RNA via iScriptTM cDNA Synthesis Kit (Bio-Rad, USA) following manufacturer's instructions. Gene expression was analyzed by qRT-PCR using MyiQ real time PCR (Bio-Rad, USA). The applied primers () were designed by Oligo software (Oligo Perfect Designer, Invitrogen, USA). The amplification was done for 40 cycles (95°C 20 sec, 60°C 20 sec, 72°C 40 sec). To verify the PCR product, melting curves were carried out in each reaction. Relative quantification of mRNA was determined by the ΔΔCt method (2−ΔΔCt formula) Citation22 by using the expression profile of the corresponding control samples as reference. Data processing and statistical analysis: Prism 6.0 (GraphPad Software, USA) was used for data plotting, non-linear regression and statistical analysis. Data are given as mean ± S.E.M. Data groups were compared using Students unpaired t-test. Results were considered statistically significant when P < 0.05.
Table 1. Designed Primers for Quantitative Real Time PCR (qRT-PCR)
Western Blot analysis
C2C12 myoblasts and MMP1 genetic engineered cells were lysed when cell density reached 70% confluency. The samples were separated on a 12% sodium dodecyl sulfate–polyacrylamide electrophoresis gel and transferred to nitrocellulose membranes used to perform immunostaining. The primary antibodies rabbit polyclonal anti-MMP1 (ab38924, Abcam, MA), at concentrations of 1:2,000 were developed for 1 hr at room temperature. Mouse anti-β-actin and anti-glyceraldehyde-3-phosphate dehydrogenase (Sigma, St. Louis, MO) were used for protein quantification and were diluted to 1:5,000. The secondary anti-rabbit horseradish peroxidase (Pierce, Rockford, IL) was used at a concentration of 1:8,000 for 1 hr. Peroxidase activity was determined by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ), and the positive bands were detected on X-ray film. Northern Eclipse software v.6.0 (Empix Imaging, Mississauga, Canada) was used to evaluate all results.
Animal experiments
All animal experiments were approved by the Children's Hospital of Pittsburgh. The University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) at the authors’ institution approved all experimental protocols (No. 15/03).
Myoblast transplantation
Eight-week-old mdx/SCID mice (C57BL/10ScSn-DMDmdx crossed with C57BL/6J-Prkdcscid/SzJ) were used in this application.Citation23,24 LacZ-pre-labeled control C2C12 myoblasts (1 × 105) or MMP1 transfected myoblasts were diluted with 5 μl of PBS and separately injected into the left and right gastrocnemius muscles (GMs) of mdx/SCID mice. Muscle tissues were harvested for histological analysis at 2 and 4 weeks after transplantation. The muscle tissues were isolated, mounted, and frozen in 2-methylbutane cooled in liquid nitrogen. Each muscle specimen was cryostat-sectioned at 10 μm for histological analysis. LacZ staining and immunohistochemistry for dystrophin (Sigma) or β-galactosidase (Abcam, Cambridge, MA) were performed. The dystrophin-positive myofibers were counted and their diameters were measured to evaluate the enhancement of MMP1 on myoblast differentiation and fusion capacities in vivo. Results were quantified and analyzed using Northern Eclipse software (Empix Imaging Inc.).
Cell systemic delivery
Attempts to repair muscle damage in DMD patients by transplanting skeletal myogenic cells directly into dystrophic muscles must deal with the issue of limited migration of these cells. Additionally, myogenic cell transplantation into the diaphragm, heart, and intercostal muscles, is highly challenging from a technical perspective. The delivery of myogenic cells to the sites of muscle lesions via the systemic circulation is a potential alternative approach to treat this disease.Citation25 We selected MMP1 transfected myoblasts and control myoblasts for systemic deliveryCitation26,27 into dystrophic skeletal muscles of mdx/SCID (dystrophic/immunodeficient, C57BL/10ScSn-Dmdmdx crossed with C57BL/6J-Prkdcscid/SzJ) mice to avoid immunorejection. The 1 × 105 of pre-labeled cells, such as retrovirus LacZ gene transferred control and MMP1 genetically engineered myoblasts, were separately injected directly into different mice at the site of tail veil with 100 μL sodium dilution.
Immunohistochemical analysis
Serial 10-μm cryostat sections were prepared using standard techniques. For immunohistochemistry, the slides were fixed with formalin (4%) for 5 minutes after LacZ staining, and then blocked with donkey serum (10%) for 1 hr. Rabbit anti-dystrophin antibody (Abcam, Cambridge, MA) was applied to the slides at a 1:300 dilution for 60 minutes at RT. The secondary antibody, goat anti-rabbit (Alexa Fluor® 488; Molecular Probes, Eugene, OR), or HRP conjugated IgG were used at a concentration of 1:2000 for 45 minutes at RT. The DAB (2%) was used to develop positive brown signals. Negative controls were performed concurrently with all immunohistochemical staining. The nuclei of the sections were revealed using DAPI staining (Sigma, St. Louis, MO), and fluorescent microscopy was used to visualize the results as described above. For the image analysis, each muscle pair (genetic and control cells) was selected similar locations of 5–7 slides (picked one in each 3 series slides) to quality immune stained results. We also selected (randomly) 5–9 locations of each slide to measure the number of dystrophin positive vs. total muscle fibers in the vision fields of the systemic delivered muscles in the mdx/SCID mice.
Statistical analysis
LacZ-positive myofibers were counted in 10 representative sections. Both the diameter and number of LacZ- and dystrophin-positive myofibers were assessed at different time points in each group. The statistical significance of differences between the various groups was determined using a t-test or one-way or 2-way analysis of variance.
Results
MMP1 plasmid constructs
Western blot analysis was used to verify the selected clone cells’ expression of MMP1 (). The supernatant from the culture containing the cloned 293 cells was collected to evaluate the titer, and subsequently transfer to C2C12 myoblasts and MDSCs. Both transferred C2C12 myoblasts and MDSC cultures expressed high levels of MMP1 (). We also discovered that there was no significant difference in the cell proliferation rate between cells with or without MMP1 gene transfection. ().
MMP1 gene transfection promoted myoblast migration
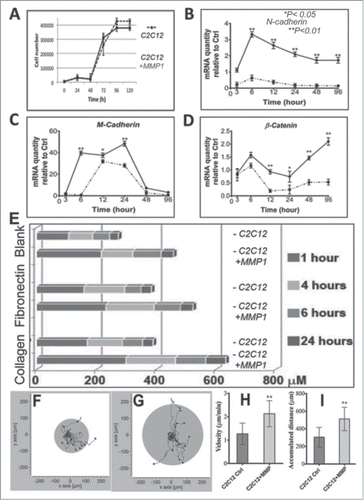
Within the cell growth curve, the MMP1 gene transfer did not influence the cell growth rate, indicating there is no effect to cell migration through cell proliferations (). We applied quantitative real-time PCR (qRT-PCR) to identify genetic modifications of MMP1 genetically engineered myoblasts in migration capacity. Our results demonstrated that MMP1 gene transfer promoted upregulation of N-Cadherin, M-Cadherin and β-Catenin, as well-known cell migration markers, when compared to the control C2C12 myoblasts (–). With artificial cell wound model,Citation18 we also tested cell migration ability at various time points after incubated for 6, 12 and 24 hrs. We observed that MMP1 gene transduction promoted myoblasts to migrate into a scratch wound faster than control C2C12 myoblasts at various time period (1, 4, 6 and 24 hrs) or on different ECM coatings (): noncoated (blank), fibronectin-coated, and/or collagen-coated flasks. In addition, we also observed increased migration rate of MMP1 transfected cells (), when compared to the control cells in the single cell migration assay (). As there is no effect in the cell growth after MMP1 transfection, our results demonstrated that MMP1 truly enhanced myoblast migration.
Figure 2. Migration assays. Those MMP1 genetic engineered C2C12 myoblasts and control C2C12 myoblasts have similar growth curve (A). Our qRT-PCR results indicated MMP1 transfer could activate migration related genes in target myoblasts; e.g. N-cadherin (B), M-cadherin (C), and β-catenin (D). An artificially cell wound model was used to identify the cell migrations as we presented before. Results in this study indicated MMP1 gene transfer accelerated migration distance of genetic myoblasts at various time periods (1, 4, 6 and 24 hrs) as well as within different conditional flasks (blanked or coated with fibronectin and collagen type I) (E). The living cell image data indicated the MMP1 genetic engineered C2C12 myoblasts (G) have greater migration speed (H) and distance (I) compare to control C2C12 myoblasts (F, H, and I). (A–D) The dotted lines correspond to control C2C12 myoblasts, and the continuous lines correspond with MMP1 transferred C2C12 myoblasts). (*P < 0.05, or **P < .01).
MMP1 gene transfer upregulates myogenic genes and enhances myoblast differentiation in vitro
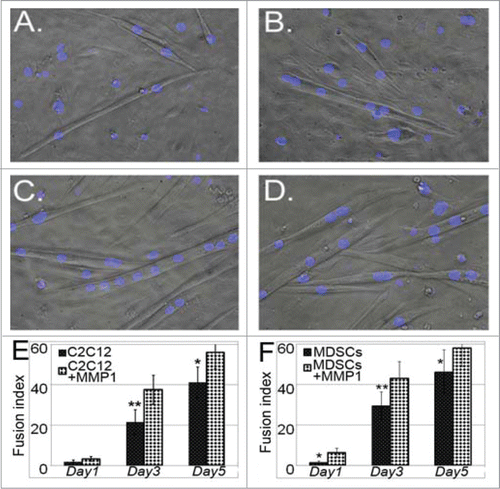
We investigated myogenic gene expression in myoblasts with or without MMP1 gene transfection under both proliferation and differentiation conditions. The differentiation process was monitored from the plated undifferentiated cells (day 1) till day 7 in differentiation medium. Our results indicated that MMP1 gene transfer upregulated myogenic genes, including Insulin Growth Factor receptor (IGFR), Muscle regeneration factor (Mrf)-4, Myogenin, Desmin and MyoD in either proliferation (–) or differentiation conditions (–). During the differentiation process most of the cells started forming myotubes; however, several cells (control or MMP1-transfected cells) in the culture did not differentiate yet, or just started forming myotubes at later time points. This was also mirrored in their gene expression profile in differentiation medium, as 2 waves of initiation of differentiation happened, first at day 3, then around day 7. We also investigated whether MMP1 gene transfection has any effects on myogenic cell differentiation. Our results showed that MMP1 gene-transfected cells (i.e., either C2C12 myoblasts or MDSCs), formed more myotubes when compared to control cells (). It is unclear what mechanism underlies MMP1s ability to promote myogenic cell differentiation; however, MMP1 triggered down-regulation of fibronectin could be one of the potential mechanisms. It is also possible that MMP1 directly affect myogenic cell fusion. Our further studies will investigate the relationship behind MMP1 expression, the level of fibronectin and myogenic cell differentiation, and the underlying mechanisms involved.
Figure 3. qRT-PCR assays. By applying the qRT-PCR technique, we screened the potential changes of myogenic genes in the genetically engineered myoblast. Gene expression levels are shown during proliferation (A–E) and myogenic differentiation (F–J) of control C2C12 myoblasts and C2C12-MMP1 transfected myoblasts. Gene expression levels were normalized to the housekeeping gene β-Actin. Data are given as mean ± SEM of 3 independent experimental runs. Statistically significant changes (*P < .05 and **P < .01) were determined by comparing to the control C2C12 myoblasts. (A–J) The dotted lines correspond to control cells, and the continuous lines correspond with MMP1 transferred cells). (*P < .05, **P < .01).
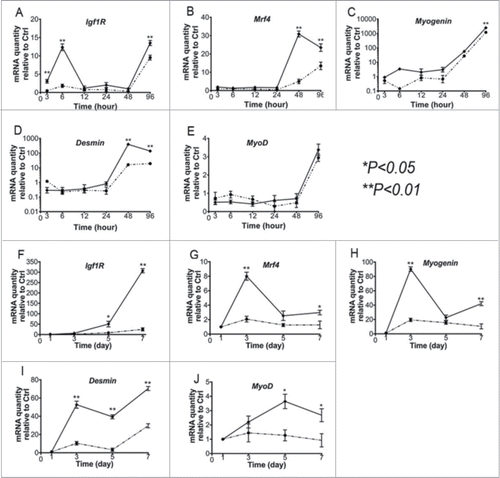
Figure 4. Myogenic differentiation assays. With low nutrient culture (muscle differentiation medium), the MMP1 gene transferred myoblasts (C) and muscle stem cells (D) illustrated stronger myogenic differentiation capacities eg. the myotube fusion index, compared to non-gene transferred control C2C12 myoblasts (A) and stem cells (B). The formed myotubes were also greater and larger in genetic cells compared to control cells (E and F). (*P < .05 or **P < .01).
MMP1 gene therapy improves myoblast transplantation efficacy
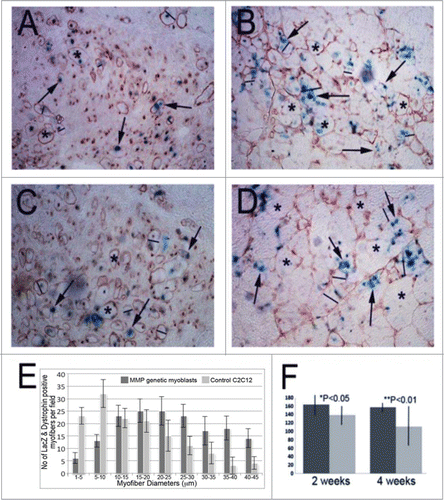
To investigate whether these MMP1-expressing cells are able to enhance engraftment efficacy in the skeletal muscle of mdx/SCID (a dystrophic/immunodeficient mouse model) mice,Citation23,24 we used a retrovirus vector to transfer the LacZ marker gene (LacZ only express in nuclei of the target cells as shown by our previous reports Citation12,16) into MMP1 transfected C2C12 myoblasts as well ascontrol C2C12 myoblasts. These cells were then transplanted into the skeletal muscles of left and right legs of the same mdx/SCID mice, respectively. We detected both LacZ-positive (blue; see arrows in –) and dystrophin-positive (brown; see asterisks; –) myofibers in the host muscle at 2 weeks () and 4 weeks () after cell transplantation. We discovered that the MMP1-expressing C2C12 myoblasts showed improved fusion/differentiation () than the control C2C12 myoblasts () into muscles’ graft at both 2 and 4 weeks after cell transplantation. We also measured the number and the diameter of LacZ-positive and dystrophin-positive myofibers in the muscles that received injection of either MMP1-expressing or control cells. Results showed that the MMP1 expressing C2C12 myoblasts not only fused into larger muscle fibers but also increased the number of dystrophin positive muscle fibers significantly () within cell implantation sites. These results indicated that MMP1 may potentially exert its beneficial effects through the paracrine mechanism.
Figure 5. Cell intramuscular implantation and engraftment in mdx mice. With immunohistochemical staining for dystrophin in the cell implanted skeletal muscle of mdx mice, we detected the dystrophin positive myofibers in both of MMP1 genetically engineered C2C12 myoblasts (C and D) and control C2C12 myoblasts (A and B) at 2 weeks (A and C) and 4 weeks (B and D) after intramuscular injections. With measuring the dystrophin positive myofibers, we found the genetically engineered myoblasts formed larger and greater in numbers compared to control C2C12 myoblasts (E and F). (The average is illustrated with standard errors: *P < .05, or **P < .01).
MMP1 gene transduction increases systemic myoblast migration in vivo
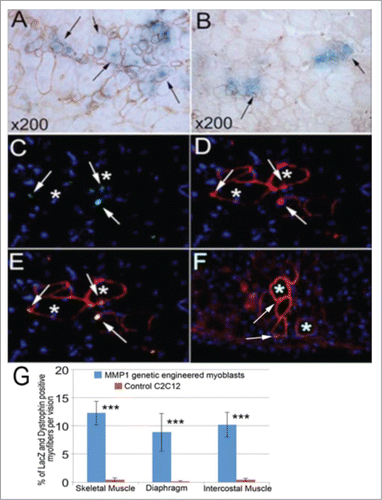
Equal quantities of MMP1+/LacZ+ C2C12 myoblasts and control LacZ+ C2C12 myoblasts (1 × 105 cells) were injected into the tail veins of different mdx/SCID mice, respectively. At various time-points (1, 2, and 3 weeks) after injection, the mice were sacrificed, and the muscle tissues including skeletal muscles, intercostal muscles, and diaphragm muscles were harvested for histological analysis. In the mice that received MMP1+/LacZ+ C2C12 myoblasts, LacZ+ myofibers were colocalized with dystrophin+ myofibers in these muscles at 2 weeks after injection (). Fewer LacZ+/dystrophin+ myofibers were detected in the skeletal muscle of the mice that received control LacZ+ C2C12 myoblasts injection (arrows, ). To investigate the distribution myoblasts that were injected systemically into mdx/SCID mice, immunohistochemistry was performed to examine the co-localization of the LacZ marker and dystrophin genes in the host diaphragm. Results show that neither LacZ+ (β-galactosidase) nor dystrophin+ myofibers were found in the diaphragm muscle following systemic delivery of the control Lac Z+ C2C12 myoblasts. However, LacZ+ myofibers (green, arrows; ) that were co-localized with dystrophin+ myofibers (red, asterisks; ) were fund within the diaphragm of mdx/SCID mice after systemic injection of MMP1+/LacZ+ C2C12 myoblasts. Similarly, myofibers that were positive for LacZ (green, arrows; ) and dystrophin (red, asterisks; ) were observed within the intercostal muscles following MMP1+/LacZ+ C2C12 myoblast injection but not the control Lac Z+ C2C12 myoblasts. We detected significantly more of MMP1+ myoblasts that fused with local muscle fibers (), suggesting the enhanced migratory ability of MMP1 transfected cells. These results support the notion that MMP1 is able to increase myoblast migration and may facilitate donor cell distribution into different tissues following systemic delivery. Taken together, results from our study support the notion that MMP1 facilitates myogenic cell migration.
Figure 6. Systemic delivery through tail vein and cell migration assays. In the tail vein injected mice, we detected large number of LacZ positive cells in the liver tissue (not shown) of injection with both genetic and control C2C12 myoblasts. However, we found more LacZ positive myofibers in the skeletal muscle of genetically engineered C2C12 myoblasts injected mice (A) compared to the control C2C12 myoblasts injected mice (B). With dystrophin immunostaining, we also confirmed that most of the dystrophin positive myofibers also expressed LacZ (A and B), but the dystrophin positive myofibers were smaller and fewer in the muscle of control cell injection (B) compared to genetic myoblasts injected mice (A). We also detected some β-gal (LacZ) (C and E) and dystrophin positive myofibers (D and E) in the diaphragm, and in the intercostal muscle (F) of the genetic myoblast injected mice, but no similar discovery was found in the control injected mice. The general measurement of LacZ positive and dystrophin expressed myofibers indicated the significance of cell migration capacity within the genetically engineered myoblasts (G). (***P < .001).
Discussion
Duchenne muscular dystrophy (DMD) is a devastating X-linked muscle disorder characterized by progressive muscle weakness that is caused by a lack of dystrophin expression in the sarcolemma of muscle fibers. Restoration of functional dystrophin in DMD may be achieved by gene therapy or the transplantation of myoblasts expressing wild-type (wt) dystrophin.Citation2,28-30 Additionally, the delivery of normal or genetically modified myogenic cells (including myoblasts and stem cells) has been explored as a therapeutic option in humans, but with limited success.Citation1,6,31 In essence, myogenic cells carrying wt dystrophin fuse and provide preexisting dystrophic muscle fibers with functional dystrophin protein expression. Myogenic cell transplantation (including stem cells, satellite cells, and myoblasts) has been well studied and considered as a potential strategy to rescue damaged skeletal muscle from injury and/or disease; it may be particularly effective in patients suffering from Duchenne and Becker Muscular Dystrophies.Citation32-34
Two issues, poor cell survival and migration of grafted cells outside the injection site following transplantation have significantly hindered the overall application of this technology.Citation35,36 The cell death has been well studied and significantly improved by the prevention of an immune-response (rejection).Citation1,37,38 The donor cell migration ability also plays a key role in effectively introducing the dystophin gene to the diseased muscles. However, only limited progress has been made in this regard. Currently, repeated cell injection or multiple injection sites have been used to approach the clinic goals but may results in pain, discomfort, and scar tissue formation at the sites of injection.Citation1,4,35,39 Therefore, the development of a novel therapeutic approach to enhance the migratory properties of the transplanted cells may greatly enhance the therapeutic efficacy and improve patient's quality of life.
MMP1 is a collagen-digesting enzyme that can eliminate existing fibrous scar (built by collagen type I and III) in different tissues including skeletal muscle tissues, but will not affect the collagen type IV, which forms the basal lamina and maintains adult muscle structures.Citation42 Previous studies indicated that MMP1 treatment facilitated myoblast migration and fusion Citation43,44 in a way similar to MMP-9 treatment.Citation45 Recent study also reported that the inhibition of MMP-9 stimulates myofiber regeneration and improves engraftment of muscle progenitor cells in the dystrophic muscles.Citation46 Thus, MMPs may improve muscle regeneration through direct or indirect pathways. The results from the current study corroborate with our previous report that MMP1 treatment improve muscle healing by enhancing myoblast differentiation and migration in vitro and in vivo.Citation16,18 Our previous study also showed that the beneficial effects of relaxin on muscle injury repair is MMP mediated.Citation47 We detected in this study that MDSCs themself also contain certain level of MMP1, which expresses as similar as in the MMP1 genetic engineered MDSCs (). We also found that MMP1 gene transfer has no significant effect in natural MDCS’ characteristics, including the study of muscle engraftment after intramuscular injection into mdx mice, indicating that MMP potentially effects stem cell behaviors in a dose dependent manner. In fact, limitation of MMP1 expression by its inhibitor could result in decreasing muscle progenitors and slow muscle healing process with fibrosis formation.Citation19 Our recent study also indicated the application of MMP1 is able to accelerate murine digit regeneration with less fibrous scar, partially because of its role in anti-fibrosis.Citation48 These studies made MMP1 an ideal candidate in improvement of myoblast transplantation. Our current study used a retrovirus vector to transfer MMP1 gene into C2C12 myoblasts in order to extending MMP1 biological lifetime prior to cell transplantation into animals. Our results demonstrated that MMP1 gene expression enhanced myoblast migration and fusion in the skeletal muscle of mdx/SCID mice in vivo. More importantly, our study showed that it is plausible to expand the distribution of transplanted myogenic cells in several muscle types of the mdx/SCID mice through systemic injection (blood stream), including intercostal muscles and diaphragms. This observation is of great importance as it has been a challenge clinically to deliver cells to these muscle types. Our study suggests the systemic injection of MMP1 expressing cells could be promising for treating DMD patients.
In summary, our MMP study in skeletal muscle system has shown that MMP1 promotes myoblast differentiation, migration and improves myoblast engraftment in vivo in mdx/SCID mice model. The overall goal of our study is to identify a novel technique to apply to improve the delivery of dystrophin gene into the dystrophic muscle fibers. We expect that this technical approach would increase the clinical applicability of myogenic transplantation therapy in injured and diseased skeletal muscles.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
HYP, KV, FWM, TTL, and YL designed research; HYP, KV, FWM, TTL, and LY performed research; HYP, KV, FWM, TTL, LY and YL analyzed data; HYP, KV, FWM, TTL, LY, YW, JH, CSC, KPL, and YL discussed results; HYP, KV, FWM, TTL and YL wrote the paper.
Acknowledgments
The authors would thank Ms. Mia Jefferson, Ms. Kiley Murray for their technical assistance, Dr. Bridget Deasy for live-cell image analysis, and Dr. Thomas Payne and Dr. Makuto Ikezawa for their help on plasmid construction.
References
- Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med 1997; 3:970-7; PMID:9288722; http://dx.doi.org/10.1038/nm0997-970
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 1989; 337:176-9; PMID:2643055; http://dx.doi.org/10.1038/337176a0
- Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, et al. Corrigendum: mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2013; 494:506; PMID:23426261; http://dx.doi.org/10.1038/nature11976
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugré FJ, Sylvain M, Lachance JG, Deschênes L, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol 2006; 65:371-86; PMID:16691118; http://dx.doi.org/10.1097/01.jnen.0000218443.45782.81
- Skuk D, Roy B, Goulet M, Chapdelaine P, Bouchard JP, Roy R, Dugré FJ, Lachance JG, Deschênes L, Hélène S, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther 2004; 9:475-82; PMID:15038390; http://dx.doi.org/10.1016/j.ymthe.2003.11.023
- Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant 1993; 2:99-112; PMID:8143083
- Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R. Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord 1999; 9:28-33; PMID:10063832; http://dx.doi.org/10.1016/S0960-8966(98)00093-5
- Zanotti S, Saredi S, Ruggieri A, Fabbri M, Blasevich F, Romaggi S, Morandi L, Mora M. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol 2007; 26:615-24; PMID:17662584; http://dx.doi.org/10.1016/j.matbio.2007.06.004
- Li Y, Cummins J, Huard J. Muscle injury and repair. Curr Opin Orthop 2001; 12:409-15; http://dx.doi.org/10.1097/00001433-200110000-00008
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 2002. 84-A:822-32; PMID:12004029
- Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol 2002; 161:895-907; PMID:12213718; http://dx.doi.org/10.1016/S0002-9440(10)64250-2
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 2004; 164:1007-19; PMID:14982854; http://dx.doi.org/10.1016/S0002-9440(10)63188-4
- Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002; 66:19-32; PMID:12228918; http://dx.doi.org/10.1002/bip.10201
- Carmeli E, Moas M, Reznick AZ, Coleman R Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 2004; 29:191-7; PMID:14755482; http://dx.doi.org/10.1002/mus.10529
- Lehto M, Sims TJ, Bailey AJ Skeletal muscle injury–molecular changes in the collagen during healing. Res Exp Med (Berl) 1985; 185:95-106; PMID:3992061; http://dx.doi.org/10.1007/BF01854894
- Bedair H, Liu TT, Kaar JL, Badlani S, Russell AJ, Li Y, Huard J. Matrix metalloproteinase-1 therapy improves muscle healing. J Appl Physiol 2007; 102:2338-45; PMID:17551103; http://dx.doi.org/10.1152/japplphysiol.00670.2006
- Kaar JL, Li Y, Blair HC, Asche G, Koepsel RR, Huard J, Russell AJ. Matrix metalloproteinase-1 treatment of muscle fibrosis. Acta Biomater 2008; 4:1411-20; PMID:18440885; http://dx.doi.org/10.1016/j.actbio.2008.03.010
- Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol 2009; 174:541-9; PMID:19147819; http://dx.doi.org/10.2353/ajpath.2009.080509
- Bellayr I, Holden K, Mu X, Pan H, Li Y. Matrix metalloproteinase inhibition negatively affects muscle stem cell behavior. Int J Clin Exp Pathol 2013; 6:124-41; PMID:23329998
- Bellayr I, Mu X, Li Y. Biochemical insights into the role of matrix metalloproteinases in regeneration: challenges and recent developments. Future Med Chem 2009; 1:1095-111; PMID:20161478; http://dx.doi.org/10.4155/fmc.09.83
- Li Y, Pan H, Huard J. Isolating stem cells from soft musculoskeletal tissues. J Vis Exp 2010; PMID:20644509; http://dx.doi.org/10.3791/2011
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262
- Li Y, Li J, Zhu J, Sun B, Branca M, Tang Y, Foster W, Xiao X, Huard J. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther 2007; 15:1616-22; PMID:17609657; http://dx.doi.org/10.1038/sj.mt.6300250
- Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther 2009; 17:1788-98; PMID:19603004; http://dx.doi.org/10.1038/mt.2009.136
- Bachrach E, Perez AL, Choi YH, Illigens BM, Jun SJ, del Nido P, McGowan FX, Li S, Flint A, Chamberlain J, et al. Muscle engraftment of myogenic progenitor cells following intraarterial transplantation. Muscle Nerve 2006; 34:44-52; PMID:16634061; http://dx.doi.org/10.1002/mus.20560
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC.. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999; 401:390-4; PMID:10517639
- Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 2007; 15:1086-92; PMID:17440445
- Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A, Wolff JA, Davies KE. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature 1991; 352:815-8; PMID:1881437; http://dx.doi.org/10.1038/352815a0
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51:919-28; PMID:3319190; http://dx.doi.org/10.1016/0092-8674(87)90579-4
- Watt DJ, Lambert K, Morgan JE, Partridge TA, Sloper JC. Incorporation of donor muscle precursor cells into an area of muscle regeneration in the host mouse. J Neurol Sci 1982; 57:319-31; PMID:6761411; http://dx.doi.org/10.1016/0022-510X(82)90038-7
- Partridge TA. Stem cell route to neuromuscular therapies. Muscle Nerve 2003; 27:133-41; PMID:12548520; http://dx.doi.org/10.1002/mus.10243
- Skuk D. Myoblast transplantation for inherited myopathies: a clinical approach. Expert Opin Biol Ther 2004; 4:1871-85; PMID:15571450; http://dx.doi.org/10.1517/14712598.4.12.1871
- Partridge TA. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve 1991; 14:197-212; PMID:2041542; http://dx.doi.org/10.1002/mus.880140302
- Nicholson LV, Johnson MA, Gardner-Medwin D, Bhattacharya S, Harris JB. Heterogeneity of dystrophin expression in patients with Duchenne and Becker muscular dystrophy. Acta Neuropathol (Berl) 1990; 80:239-50; PMID:2205076; http://dx.doi.org/10.1007/BF00294640
- Mills P, Lafreniere JF, Benabdallah BF, El Fahime EM, Tremblay JP. A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res 2006; 313:527-37; PMID:17156777
- Caron NJ, Asselin I, Morel G, Tremblay JP. Increased myogenic potential and fusion of matrilysin-expressing myoblasts transplanted in mice. Cell Transplant 1999; 8:465-76; PMID:10580341
- Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve 1996; 19:853-60; PMID:8965839; http://dx.doi.org/10.1002/(SICI)1097-4598(199607)19:7%3c853::AID-MUS7%3e3.0.CO;2-8
- Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med 1995; 333:832-8; PMID:7651473; http://dx.doi.org/10.1056/NEJM199509283331303
- Karpati G, Holland P, Worton RG. Myoblast transfer in DMD: problems in the interpretation of efficiency. Muscle Nerve 1992; 15:1209-10; PMID:1406777; http://dx.doi.org/10.1002/mus.880151016
- Listrat A, Picard B, Geay Y. Age-related changes and location of type I, III and IV collagens during skeletal muscle development of double-muscled and normal bovine foetuses. J Muscle Res Cell Motil 1998; 19:1-14; PMID:9477372; http://dx.doi.org/10.1023/A:1005305824838
- Ozawa J, Kurose T, Kawamata S, Kaneguchi A, Moriyama H, Kito N. Regulation of connective tissue remodeling in the early phase of denervation in a rat skeletal muscle. Biomed Res 2013; 34:251-8; PMID:24190237; http://dx.doi.org/10.2220/biomedres.34.251
- Ahtikoski AM, Koskinen SO, Virtanen P, Kovanen V, Risteli J, Takala TE.. Synthesis and degradation of type IV collagen in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiol Scand 2003; 177:473-81; PMID:12648165; http://dx.doi.org/10.1046/j.1365-201X.2003.01061.x
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res 2000; 258:279-87; PMID:10896779; http://dx.doi.org/10.1006/excr.2000.4962
- Lafreniere JF, Mills P, Tremblay JP, El Fahime E. Growth factors improve the in vivo migration of human skeletal myoblasts by modulating their endogenous proteolytic activity. Transplantation 2004; 77:1741-7; PMID:15201676; http://dx.doi.org/10.1097/01.TP.0000131175.60047.EB
- Morgan J, Rouche A, Bausero P, Houssaini A, Gross J, Fiszman MY, Alameddine HS. MMP-9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve 2010; 42:584-5; PMID:20734311; http://dx.doi.org/10.1002/mus.21737
- Zimowska M, Olszynski KH, Swierczynska M, Streminska W, Ciemerych MA. Decrease of MMP-9 activity improves soleus muscle regeneration. Tissue Eng Part A 2012; 18:1183-92; PMID:22429194; http://dx.doi.org/10.1089/ten.tea.2011.0459
- Mu X, Urso ML, Murray K, Fu F, Li Y. Relaxin regulates MMP expression and promotes satellite cell mobilization during muscle healing in both young and aged mice. Am J Pathol 2010; 177:2399-410; PMID:20934971; http://dx.doi.org/10.2353/ajpath.2010.091121
- Mu X, Bellayr I, Pan H, Choi Y, Li Y. Regeneration of soft tissues is promoted by MMP1 treatment after digit amputation in mice. PLoS One 2013; 8:e59105; PMID:23527099; http://dx.doi.org/10.1371/journal.pone.0059105
