Abstract
A compelling amount of data is accumulating about the polyphonic role of neuronal cadherins during brain development throughout all developmental stages, starting from the involvement of cadherins in the organization of neurulation up to synapse development and plasticity. Recent work has confirmed that specifically N-cadherins play an important role in asymmetrical cellular processes in developing neurons that are at the basis of polarity. In this review we will summarize recent data, which demonstrate how N-cadherin orchestrates distinct processes of polarity establishment in neurons.
Extrinsic and Extrinsic Cues Influencing Neuronal Polarity in Vivo and Modelling Polarity in Vitro
In biology the term polarity describes a non-uniform, asymmetric organization of organs, single cells or even single cellular compartments. A tightly controlled asymmetric localization of molecules is necessary for cells to perform diverse biological processes, such as asymmetric cell division, directional cell contact, cell differentiation, cell orientation, cell migration and formation of specific sub-cellular structures.
In its broadest sense, neuronal polarity refers to the asymmetric organization of the neuron in axonal and dendritic domains as well as to the asymmetric localization of organelles and biomolecules throughout the development of the brain. Several “polarized” processes can be identified during neuronal development, including the generation of neurons from asymmetric divisions, migration to a final homing position, axon/dendrite formation and asymmetric trafficking. Ultimately, in neurons polarity governs the subcellular organization of components that gives rise to either an axonal or a dendritic domain. Specific membranes, organelles, receptors and cytoskeletal components characterize these specialized domains, ensuring the correct directional flow of electrochemical information within neurons. Since polarity is crucial for the terminal differentiation of neurons and for their correct wiring, failures in polarity establishment affect the functioning of the brain and contribute to a number of diseases such as mental retardation and epilepsy.Citation1
In vivo, at least 3 polarity-related processes are required to establish the final location and wiring of neurons in mammals (). Excitatory pyramidal neurons clearly exemplify these processes. Pyramidal neurons originate from precursors localized in the ventricular and in the subventricular zone of the dorsal telencephalon (for a review see refs.Citation2-4). Upon radial migration, pyramidal neurons move to the superficial layer of the developing cortical plate populating the cortex within discrete layers.Citation5 During their journey pyramidal neurons first undergo a “mitotic polarity” step, which coincides with the division of a neuronal precursor that gives rise to a postmitotic neuron. The second step corresponds to the “migration polarity,” which is necessary to orient and determine the movement of these neurons to their final destination. The third process is the establishment of the definitive “axon/dendrite polarity” which starts during migration and is manifested once neurons have reached their final destination.
Figure 1. Schematic representation of polarity stages in pyramidal neurons in vivo and hippocampal neurons in vitro. (A) Excitatory cortical neurons derive from a radial glia cell, which divides asymmetrically giving rise to a supporting cell (gray) and a neuron (red). Following division, neurons have a polar phenotype with a leading pole (LP) oriented toward the pia and a trailing pole (TP) oriented toward the ventricular/subventricular zone (VZ/SVZ). In the intermediate zone (IZ) some cells show a multipolar stage characterized by several short processes that are not aligned with the radial glial fibers,Citation7 which may initiate axon extension.Citation8-12 Finally, neurons re-acquire a bipolar axis with the LP directed toward the pia, completing their migration to their final destination in the cortical plate (CP). Acquisition of mature polarized features, including the terminal differentiation of dendrites and somatic compartment is represented in green. (B) Hippocampal neurons are dissected from embryonic rodent hippocampus, trypsinized and plated on coverslips grown with the help of a glial feeder layer. Shortly after plating round neurons have a polarized organization of the cytoplasm, with the organelle pole not fixed in one direction (intracellular polarity; stage 0). An adhesion-dependent mechanism stabilizes the organelle pole and supports the sprout of the first neurite (pre-polarity stage 1, monopolar neuron). Note that the first neurite has the highest chances to become the axon at later stages. Subsequently, neurons acquire a bipolar axis with the growth of a second neurite from the pole in the opposite side of the first sprout (bipolar stage 2). Several other neurites grow, giving rise to a multipolar phenotype (multipolar stage 2). During the multipolar stage 2, rapid growth occurs from one of the 2 predisposed neurites of the bipolar axis (stage 3).Citation14-16
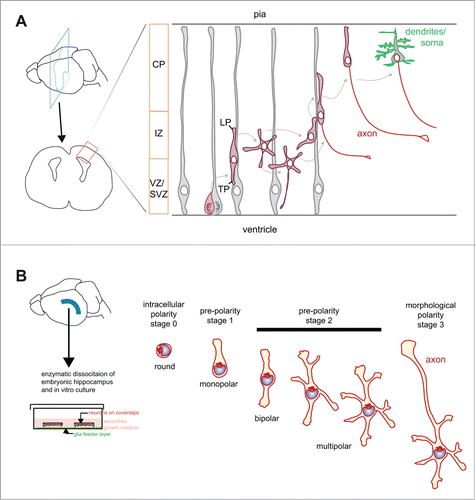
Not all neurons follow the same polarity phases. In some cell types the “migrational polarity” is tightly maintained and gives rise to the “axon-dendrite polarity,” as in the case of the developing retinal amacrine cells.Citation6 In pyramidal neurons the first bipolar, polarized stage is transiently substituted by a multipolar stage in which cells form several short processes that are not aligned with the radial glial fibers.Citation7 Neurons re-acquire a bipolar phenotype later on, with the leading process –the apical dendrite- directed toward the pia and the axon toward the ventricle, and complete their journey to their final destination. The axon forms either during the multipolar stage in the intermediate zone or during migration when neurons enter the cortical plate as bipolar neurons.Citation8-12
The presence of at least 3 polarity events occurring in an intermingled manner in situ complicates the interpretation of pharmacological and genetic results aimed at defining the basic determinants of polarity. As an example, genetic ablation of SAD-A and SAD-B, the mammalian orthologs of a kinase discovered in C. elegans, affects both “migrational polarity” and “axon-dendrite polarity” impairing cortical layer formation, axon orientation and showing a starburst morphology, where axons are difficult to distinguish from dendrites.Citation13 Since the 3 polarity processes overlap in several genetic and mechanistic aspects, manipulating one of the polarity steps can affect the others. Furthermore, polarity studies in vivo are complicated by the fact that neurons in the developing brain are embedded in an intricate 3-dimensional environment composed by cells and extracellular matrix components that contain several directional cues, functioning as extracellular variables that compete with the intrinsic cell polarity pathways.
Notably, the process of axon formation, and more specifically the decision of conferring axonal identity to one specific emerging neurite, is one of the most striking and more thoroughly studied events of neuronal polarization. Historically, to simplify the interpretation of experiments, axon formation has been largely modeled in culture, where neurons do not migrate and their morphology can be studied in great detail. Neurons in culture differentiate in a very stereotyped manner.Citation14 Following dissociation from E16-E18 mouse or rat hippocampus cells appear round (stage 1). In 4–24 hours neurons extend several morphologically similar minor neurites that elongate and retract in a dynamic cycle not showing net growth (stage 2). After 24–36 hours, one of the minor neurites rapidly elongates and undergoes transformation into an axon (stage 3). This model set the emergence of the axon at the transition between stage 2 and 3, when one of the minor neurites elongates and phenotypically becomes the axon ().
More recently this model has been updated () when it was shown that the first neurite that arises from round cells has the highest probability to become the axon during subsequent development.Citation15 Furthermore, the final axon-dendrite identity is not stochastically determined, but it is pre-defined by a bipolar axis.Citation16 To continue with the previous classification of stages, the immediate post-mitotic neuron corresponds to stage 0, which already has a polarized cytoplasm. The stage in which the neuron grows its first sprout from the organelle pole site is named stage 1. Subsequent stages are comparable with the previous classification. Under this perspective, the establishment of axonal polarity has 2 new significant steps. The first step corresponds to the processes occurring immediately after mitosis, at the transition between stage 0 and stage 1, when the first bud forms. The second important step corresponds to the transition from the bipolar stage 2, when the neuron has previously defined its preferred, intrinsic axis of growth, to growth of the future axon. In fact, at the bipolar stage, even though the 2 processes are morphologically indistinguishable, the neuron “knows” which of them has the highest chances to become the axon.
The above evidences suggest that an intrinsic program of polarity is already present in the round, postmitotic neurons. In support of this conclusion, in vitro axon specification occurs in the absence of extracellular asymmetrical cues.Citation14,17,18 It was long hypothesized that in a homogenous environment, free of asymmetric cues (such as a clean coverslip), the selection of a neurite to undergo axonal transition would be stochastically determined by intrinsic cellular factors. Eventually, extracellular cues can interfere with the intrinsic pathways. Extrapolating to the in vivo scenario, neuronal polarization appears as an extremely complex process critically defined by the numerous cell-cell adhesion and soluble cues present in the 3 dimensional environment of the brain tissue.Citation19-26 Although the relative weight of intrinsic/extrinsic signals is difficult to be put into perspective, recent work show that adhesion molecules, and in particular N-cadherin can instruct the intracellular polarity program and establish the polarity axis of developing neurons.
As we will see, cadherins are a family of molecules able to integrate extracellular cues with adhesion and intracellular signaling pathways. In this review we highlight the importance of N-cadherin, a classical member of the cadherin superfamily family, which combines environment sensing through adhesion and modulates neuronal polarity. We start by introducing the cadherin family and in particular classical cadherins, focusing on ways to regulate their surface availability and signaling capacities; later we explore their ability to influence polarity in general, and in the last paragraph we show how N-cadherin specifically regulates the establishment of neuronal polarity.
Cadherins: Linking Adhesion to Signal Transduction
Cadherins are a large protein family, and all members have in common several extracellular so called cadherin motifs which are important for calcium-dependent cell adhesion.Citation27 The initially identified “classical cadherins” are the prototypic cell adhesion molecules, while many other members have diverse signaling functions.Citation28 Importantly, many cadherins are specifically expressed in the nervous system and have important roles in brain function and development.
Given the large number of excellent reviews on the superfamily of cadherins and their specific signaling and physiological functions,Citation28,29 we will here concentrate on those classical cadherins, which were recently described to play a role in neuronal polarity control during development.
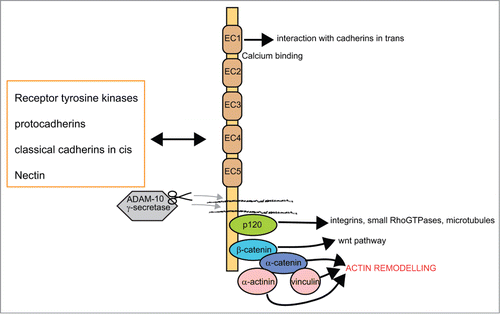
Classical cadherins are type I transmembrane proteins and display 5 extracellular cadherin motifs. The extracellular cadherin domains are stabilized by calcium and are thus able to interact in cis and in trans with other cadherins, typically in a homophilic manner. While the trans-interactions mediate cell-cell adhesion, it is thought that the cis/lateral interactions support the clustering of cadherins at junctions.Citation30 In theory this interaction of cadherins in cis and/or trans could create different levels of adhesiveness depending on the local cadherin availability. The trans-binding leads to the binding of N-cadherin to the cytoskeletonCitation31 and the reorganization of the actin cytoskeleton, which induces changes in cell morphology and motility.Citation32 For the interaction with the cytoskeleton and in order to trigger strong adhesion, the intracellular domain is essential. This domain contains binding sites for p120 catenin and β-catenin, the latter binding to α-catenin, which then directly mediates the interaction to the cytoskeleton. The interaction with the cytoskeleton is crucial for regulating cell polarity and is mediated by direct interaction of a catenins with actin or with actin and microtubule binding molecules or Rho GTPases (see list of catenin binding partners in ref.Citation28; see scheme of interactors in ).
Figure 2. Schematic representation of domains in classical cadherins, main interactors and most relevant signaling pathways. Classical cadherins mediate homophilic interactions with other cadherins in the extracellular space, while intracellularly they organize the actin cytoskeleton and integrate several signaling pathways. The extracellular domains of cadherins are characterized by the repetition of several copies (5) of the cadherin motif, which mediates cell adhesion in a Ca2+ dependent manner.Citation27 Several interactions can be established with receptors and other adhesion molecules (for a review see refs.Citation28,29). Proteolytic processing of cadherin is regulated by the gamma-secretase/ADAM complex and mediates the release of intracellular and extracellular domains.Citation46 The intracellular domain of cadherin interacts with p120-catenin and with the β-catenin core complex. The protein p120-catenin stabilizes adhesions and regulates interactions with other adhesion molecules and membrane remodelling complexes (for a review see ref.Citation64). The core β-catenin complex has the dual function of regulating both adhesion and gene transcription. Adhesion is mediated by the interaction with α-catenin, which contacts actin and vinculin. These interactions are at the basis of actin/cytoskeletal remodelling events. Beta catenin is also implicated in the activation of wnt signaling pathways (for a review see ref.Citation65).
Classical Cadherins in Cell Polarity
Adhesion is an efficient way to create asymmetry by clustering molecules in adhesion complexes and recruiting interactors in their microenvironment. In vitro cadherin cell adhesions can be studied in a spatial limited area, e.g. contacting only one pole of a cell by means of patterned substrates.Citation33 Similar results can be achieved by severing cell-cell interactions just at one pole, for instance by removing mechanically cells with a scratch.Citation34 Wound healing assays revealed for instance that following the wound kidney epithelial cells reposition their centrosome and orient their migration axis in the direction of the cell-free areas, in an E-cadherin, actin and cdc42-dependent manner.Citation35 The intracellular pathways mediating this organelle polarization seem to differ between cell types, since for instance hippocampal neurons reorient the centrosome toward, and not away, from N-cadherin contact sites in a PI3-kinase, actin and microtubule dependent manner.Citation22 Another study in dissociated cerebellar granule cells showed that E-cadherin recruits the centrosome in GAP-43 dependent manner toward the extrinsically applied E-cadherin substrate.Citation36 In a recent study using mouse endothelial fibroblasts growing on micro-patterns where one pole of the cell is in contact with another cell and the other in cell-ECM contacts, it was shown that N-cadherin dependent engagement of p120 suppresses integrins near the N-cadherin junction, indirectly promoting the activation of PI3-kinase and Rac at the cell-free end.Citation33 In this same work, it was shown that N-cadherin binding to β-catenin at the cell-junction side promotes the accumulation of myosin light chain and actin filaments. Those data suggest that N-cadherin regulates the subcellular distribution of diverse signaling molecules through different effectors.Citation33
How in vivo a local cadherin surface expression and thus polarized adhesion and signaling are regulated is to date an open question. One possibility is via cadherin expression control, at the transcriptional level.Citation37 As a matter of fact, the rate of adhesiveness depends on the relative concentration of cadherins on the different cell surfaces (similar concentration in different, opposing, cells result in more efficient adhesiveness) which could lead to a preferential adhesion between certain cells within a tissue.Citation38 In addition, differences in adhesiveness could be obtained through the local control of cadherin within different cells. An efficient means of local control is via regulated and directional membrane remodelling. Endocytosis of N-cadherin molecules was described to be regulated in migrating neurons by rab7 and 11Citation39 or via β-catenin in synapses.Citation40 In addition, the lateral diffusion and recruitment of cadherins can be influenced by the interaction with other adhesion systems such as nectinsCitation41 or protocadherins in a synergisticCitation42 or antagonistic manner.Citation43 Furthermore, spatially-restricted adhesion can be obtained in a rapid manner without large changes in the cadherin complexes via an “inside out signaling.” In this regard, it was shown that growth factors can change intracellular signaling and affect adhesiveness by changing the interaction of cadherin with catenins either via direct tyrosine phosphorylation of α-catenin and p120 cateninCitation44 or via other targets of tyrosine kinases which indirectly influence cadherin mediated adhesion (for a review see ref.Citation45). Moreover, localized cadherin changes can be mediated by cadherin-catenin complexes interacting with receptor-type tyrosine kinases such as FGF receptors and EGF receptors outside of adhesion complexes.Citation28 This last mechanism links cell-cell adhesiveness of neurons with the chemical cues normally present in a graded manner in situ. Yet one additional mechanism for the regulation of cell surface N-cadherin levels is proteolytic processing of the full-length mature N-cadherin protein by ADAM10. This proteolysis has a dual effect: 1) it releases a soluble ectodomain potentially acting as a signaling molecule in a long-distance range and 2) it generates a 40 kDa C-terminal membrane-bound fragment which is further processed by the γ-secretase into a 35 kDa intracellular fragment. This fragment participates in the regulation of gene expression by redistributing β-catenin.Citation46
In summary these examples illustrate the multiple ways by which classical cadherins can receive signals to modulate adhesiveness in a spatially defined way, highlighting the complex levels of regulation of the cadherin pathways and, in the end, the precise spatiotemporal regulation of cadherin signaling that ought to occur to assure proper brain development.
Cadherins in Neuronal Polarity and in Brain Development
The attention toward a role of cadherins in the organization of the developing cortex derived from the fact that cadherins are expressed very early in development in the brain,Citation47 and that many cadherin genes are linked with neuropsychiatric disorders (for a review see ref.Citation48). In fact, a number of publications show that cadherins are involved in neural tube formation and regionalization, in neuronal migration and polarity, axon outgrowth, neural circuit formation, spine morphology, synapse formation and synaptic remodelling (for a review see refs.Citation28,29,37).
As previously mentioned, pyramidal neurons during the acquisition of their polarity in vivo migrate through a number of different extracellular environments and encounter different types of cells. Thus, local cadherin signals, which could support polarization, are likely to act for a very restricted period of time and in a precisely spatially-defined way, positively or negatively regulating adhesiveness. This continuous remodelling of adhesion through signaling is essential in order to actively maintain polarity and to support migration. We next summarize recent work showing how the classical type I cadherin N-cadherin is involved specifically in the establishment of neuronal polarity ().
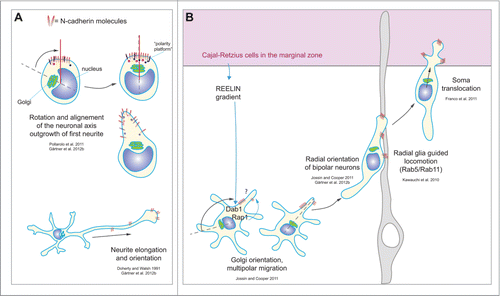
Figure 3. Schematic representation of neuronal polarity steps regulated by N-cadherin during different developmental steps. (A) The clustering of N-cadherin molecules in round neurons is able to recruit the Golgi complex and the MTOC.Citation22,52 In dissociated neurons N-cadherin promotes orientation and elongation of neurites.Citation22,49 (B) In vivo during the establishment of polarity in pyramidal neurons, N-cadherin orients the Golgi complex in multipolar neurons in a Dab1-Rap1 dependent mechanism that is induced by a reelin gradient established by the Cajal-Retzius cells in the marginal zone.Citation59 In bipolar neurons N-cadherin contributes to the radial orientation that is necessary for migration,Citation22,59 and supports the radial glia locomotion of neurons mediated by Rab5-Rab11 membrane rearrangement.Citation39 Once neurons reach the Cajal-Retzius cells in the marginal zone, N-cadherin promotes soma translocation.Citation58
Axon specification and outgrowth
N-cadherin triggers neurite outgrowth in vitroCitation49 and in vivo,Citation50 suggesting that its polarized presence could master polarized growth. Indeed, dissociated hippocampal neurons which were cultured on patterned substratesCitation21 using alternating stripes of N-cadherin and poly-L-lysine always orient their axon toward the N-cadherin substrate side.Citation22 One possible mechanism underlying this substrate-specific outgrowth could be the proposed molecular clutch mechanism, which couples actin flow with N-cadherin adhesions.Citation51
However, as mentioned earlier, neuronal polarity in vitro is defined well before axon outgrowth starts, as early as during the time of sprouting of the first neurite, and even earlier.Citation15 In order to understand whether N-cadherin could also influence those early stages of axon determination, freshly dissociated hippocampal neurons were cultured on alternating stripes of N-cadherin and poly-L-lysine.Citation22 This study showed that indeed neurons extend the first neurite from the N-cadherin contacting site.Citation22 This study also revealed that intracellular organelles such as centrosome and the Golgi are recruited to the first neurite after this has formed. A similar polarization was observed in sensory neurons in Drosophila in vivo, where a cadherin-landmark was formed shortly after neuron generation, followed by the recruitment of the centrosome.Citation52 Those data show that neuronal polarization is triggered by cadherin and one could assume that this cadherin cluster is triggered by extrinsic cues, most likely a homophilic interaction with cadherins on neighboring cells. Although being a logic assumption, this view cannot explain that neurons can polarize in complete isolation, in the absence of any asymmetric cell-cell contacts.Citation14 This in fact indicates that a cell autonomously defined polarity signal, for example the N-cadherin cluster, can suffice to trigger growth signaling. In agreement with this idea, N-cadherin was found to be polarized even before cell attachment in dissociated neurons.Citation22 Moreover, the data in sensory neurons in Drosophila pupae suggest that the cell autonomous accumulation of cadherin could be the consequence of the inheritance of components from the last mitosis cleavage zone in the new neuron.Citation52 These 2 visions could be reconciled if one would see the intrinsically defined N-cadherin cluster as a site where not only cadherins concentrate but also other growth-regulatory molecules. This type of specialized molecular asymmetry would certainly increase the probability of asymmetric growth in response to different types of external cues, irrespective of their polarized or uniform distribution (for instance BDNF). This type of intrinsically produced growth cluster or “polarity platform” could in principle support the polarization of any type of neuron: specificity could be provided by the type of environmental cue ().
N-cadherin regulates axis orientation and thus migration
The in vitro results described above suggest that extrinsic N-cadherin (when present) greatly facilitate polarized growth from the pole with an intrinsically asymmetric N-cadherin clusterCitation22,53 Recent work suggests that the intrinsic-extrinsic N-cadherin polarity signaling persists during the time when neurons become bipolar.Citation54 However it is important to understand how and whether this type of mechanism participates in the polarization and thus spatial orientation of cortical neurons in vivo. One indication that this might be the case comes from the observation that N-cadherin is polarized to the apical junctions of the neuroepithelium, suggesting that N-cadherin could play a role in the polarized organization of neuroepithelial cells and radial glia cells.Citation55 Consistently, the loss of N-cadherin in early embryos disrupted those junctions resulting in the abnormal organization of radial glia fibers,Citation55 which are crucial for proper neuron migration. Furthermore, it is known that the apical N-cadherin containing membrane remains to a large extent in the daughter radial glia cell after asymmetric cell division.Citation56 However, N-cadherin is also present in neurons in the developing cortex in the form of clusters,Citation22,55 suggesting a role during neuronal asymmetry as well. Recent studies addressed this possibility by reducing N-cadherin function in a specific and moderate manner by in utero electroporationCitation57 of dominant negative forms of N-cadherin.Citation22,58,59 With this approach the mutant protein was delivered to a subset of cells in a mosaic manner, allowing the observation of N-cadherin deficient neurons on a wild-type background. The inhibition of N-cadherin function in neurons using the dominant negative N-cadherin construct perturbed the transition of multipolar into bipolar neurons and also reduced the directional movement of migrating neurons and Golgi orientation toward the cortical plate, strengthening the notion that N-cadherin plays a role in neuronal polarity.Citation59 Furthermore, the presence of the dominant negative form of N-cadherin resulted in orientation defects in neurons in their bipolar phase, potentially altering the onset of migration along radial fibers.Citation22 However, once N-cadherin deficient neurons attached to radial glia cells, their migration speed and directionality was not changed.Citation58,59 The latter and other studies also indicated that the surface expression of N-cadherin in multipolar and bipolar neurons is regulated via small GTPases.Citation60 In turn, Rap1 seems to be regulated by Reelin, which is secreted by Cajal-Retzius cells in the marginal zone.Citation59 Therefore the Reelin signal can act as a spatial signal which, in theory, could affect N-cadherin surface signaling on the pole oriented toward the pial direction and thus in the direction of radial migration. This is also supported by: (1). the observation that the Golgi is recruited toward the pial side, possibly to prepare multipolar neurons for bipolar migrationCitation59 and (2). the observation that N-cadherin signaling for polarized growth induces Golgi translocation to the base of the growing neurite.Citation22 To date it remains to be proven whether reelin can truly polarize N-cadherin signaling in multipolar neurons in vivo. Another study suggests that N-cadherin trafficking in the developing cortex is regulated by a Rab5-dependent endocytotic and a Rab11-dependent recycling pathway and contributes to cortical neuronal migration along radial glial fibers .Citation39 However, this mechanism was not observed when a Rap1 regulated N-cadherin surface expression was studied: there only radial glia independent migration modes such as somal translocationCitation58 and multipolar migrationCitation59 but not radial glia guided locomotion were found to regulated by N-cadherin. This opens the interesting debate of how different spatio-temporal regulation of N-cadherin during development via distinct pathways could influence the response of the neurons. Moreover, it is currently not clear which is the precise mechanism used by N-cadherin to drive the transition into bipolar cells and what is the intracellular pathway that orients the cell axis ().
N-cadherin in tangential and collective migration
N-cadherin was also shown to be a key player in the establishment of polarity in inhibitory neurons, which derive from the medial ganglionic eminence (MGE) and migrate tangentially over a longer distance toward the cortical plate in the cortex.Citation61 In these in vivo and in vitro studies, MGE cells with inactivated cadherin exhibited slowed and less directed migration and polarity defects associated with abnormal actomyosin contractility. Further confirming the important role of cadherins in tangential migration, Cadherin-2 (the zebra fish homolog of N-cadherin) was shown to play a role in the migration of cerebellar granule neurons, which migrate in chain-like structures using homophilic interactions mediated by cadherin-2. Cadherin-2 transiently translocate to the front of granule cells, followed by the polarization of the centrosome in the direction of migration.Citation62 This work corroborates the idea that N-cadherin is an essential player in the different types of 3-dimensional cellular arrangements of the brain, by regulating the motility of individual cerebellar granule neurons and coordinating their collective migration behavior.
Conclusions
We have summarized the role of N-cadherin in regulating neuronal polarity and showed a number of different steps during neuron development in which N-cadherin is important.
In most studies the authors showed that one important feature of N-cadherin in polarity regulation is the recruitment of the centrosome toward the site of cadherin concentration. It is still not clear which mechanisms are engaged in this process as well as the true biological significance of centrosome positioning. Moreover, even if the polarized signaling of N-cadherin is important for the recruitment of the centrosome in vitro, it remains to be proven whether this, and what for, operates in vivo.
The data based on hippocampal neurons in vitro illustrate both that these neurons are born with a N-cadherin cluster and are still able to polarize in complete isolation, in the absence of any asymmetrically distributed exogenous N-cadherin, or any other extrinsic asymmetric cue. This, which intuitively would lead to the conclusion that the establishment of polarity is an eminently intrinsic process, may reflect that N-cadherin is just one among several molecules with ability to transduce external signals into growth (the polarity “platform”). It remains to be demonstrated the validity of this prediction and the underlying molecular mechanisms.
Another open question is whether a different regulation of N-cadherin surface availability may lead to different functions during development. For instance this could explain the distinct regulation by Rab5 and 11 during locomotion, which was not achieved by a Rap1 dependent regulation.
Another challenging open question is how the remodelling of N-cadherin clusters and adhesion points trigger migration and a continuous change in the response to the environment during migration in the distinct environments migrating neurons encounter.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gleeson JG. Neuronal migration disorders. Ment Retard Dev Disabil Res Rev 2001; 7:167-71; PMID:11553932; http://dx.doi.org/10.1002/mrdd.1024
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 2005; 6:777-88; PMID:16314867; http://dx.doi.org/10.1038/nrm1739
- Franco SJ, Muller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron 2013; 77:19-34; PMID:23312513; http://dx.doi.org/10.1016/j.neuron.2012.12.022
- Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 2014; 15:351-64; PMID:24639559; http://dx.doi.org/10.1002/embr.201438447
- Angevine JB Jr., Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 1961; 192:766-8; PMID:17533671; http://dx.doi.org/10.1038/192766b0
- Hinds JW, Hinds PL. Early development of amacrine cells in the mouse retina: an electron microscopic, serial section analysis. J Comp Neurol 1978; 179:277-300; PMID:641219; http://dx.doi.org/10.1002/cne.901790204
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci 2003; 23:9996-10001; PMID:14602813
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci 2010; 30:10391-406; PMID:20685982; http://dx.doi.org/10.1523/JNEUROSCI.0381-10.2010
- Hatanaka Y, Yamauchi K. Excitatory cortical neurons with multipolar shape establish neuronal polarity by forming a tangentially oriented axon in the intermediate zone. Cereb Cortex 2013; 23:105-13; PMID:22267309; http://dx.doi.org/10.1093/cercor/bhr383
- Namba T, Kibe Y, Funahashi Y, Nakamuta S, Takano T, Ueno T, Shimada A, Kozawa S, Okamoto M, Shimoda Y, et al. Pioneering axons regulate neuronal polarization in the developing cerebral cortex. Neuron 2014; 81:814-29; PMID:24559674
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004; 7:136-44; PMID:14703572; http://dx.doi.org/10.1038/nn1172
- Sakakibara A, Sato T, Ando R, Noguchi N, Masaoka M, Miyata T. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cereb Cortex 2014; 24(5):1301-10; PMID:23307632; http://dx.doi.org/10.1093/cercor/bhs411
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science 2005; 307:929-32; PMID:15705853; http://dx.doi.org/10.1126/science.1107403
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci 1988; 8:1454-68; PMID:3282038
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature 2005; 436:704-8; PMID:16079847; http://dx.doi.org/10.1038/nature03811
- de Anda F, Gartner A, Tsai LH, Dotti CG. Pyramidal neuron polarity axis is defined at the bipolar stage. J Cell Sci 2008; 121:178-85; PMID:18187450; http://dx.doi.org/10.1242/jcs.023143
- Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME. Development of polarity in cerebellar granule neurons. J Neurobiol 1997; 32:223-36; PMID:9032664; http://dx.doi.org/10.1002/(SICI)1097-4695(199702)32:2%3c223::AID-NEU7%3e3.0.CO;2-A
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 2000; 10:574-81; PMID:11084319; http://dx.doi.org/10.1016/S0959-4388(00)00124-0
- Dimidschstein J, Passante L, Dufour A, van den Ameele J, Tiberi L, Hrechdakian T, Adams R, Klein R, Lie DC, Jossin Y, et al. Ephrin-B1 controls the columnar distribution of cortical pyramidal neurons by restricting their tangential migration. Neuron 2013; 79:1123-35; PMID:24050402; http://dx.doi.org/10.1016/j.neuron.2013.07.015
- Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci 1999; 19:6417-26; PMID:10414970
- Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol 2000; 29:215-23; PMID:11428051; http://dx.doi.org/10.1023/A:1026515426303
- Gartner A, Fornasiero EF, Munck S, Vennekens K, Seuntjens E, Huttner WB, Valtorta F, Dotti CG. N-cadherin specifies first asymmetry in developing neurons. EMBO J 2012; 31:1893-903; PMID:22354041; http://dx.doi.org/10.1038/emboj.2012.41
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 1998; 282:1904-6; PMID:9836643; http://dx.doi.org/10.1126/science.282.5395.1904
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 2000; 404:567-73; PMID:10766232; http://dx.doi.org/10.1038/35007001
- Jossin Y, Bar I, Ignatova N, Tissir F, De Rouvroit CL, Goffinet AM. The reelin signaling pathway: some recent developments. Cereb Cortex 2003; 13:627-33; PMID:12764038; http://dx.doi.org/10.1093/cercor/13.6.627
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci 2003; 4:496-505; PMID:12778121; http://dx.doi.org/10.1038/nrn1113
- Takeichi M, Hatta K, Nose A, Nagafuchi A. Identification of a gene family of cadherin cell adhesion molecules. Cell DifferDev 1988; 25 Suppl:91-4; PMID:3061598; http://dx.doi.org/10.1016/0922-3371(88)90104-9
- Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev 2012; 92:597-634; PMID:22535893; http://dx.doi.org/10.1152/physrev.00014.2011
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 2006; 20:3199-214; PMID:17158740; http://dx.doi.org/10.1101/gad.1486806
- Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol 2012; 22:299-310; PMID:22555008; http://dx.doi.org/10.1016/j.tcb.2012.03.004
- Lambert M, Choquet D, Mege RM. Dynamics of ligand-induced, Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J Cell Biol 2002; 157:469-79; PMID:11970959; http://dx.doi.org/10.1083/jcb.200107104
- Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol 2009; 1:a003053; http://dx.doi.org/10.1101/cshperspect.a003053
- Ouyang M, Lu S, Kim T, Chen CE, Seong J, Leckband DE, Wang F, Reynolds AB, Schwartz MA, Wang Y. N-cadherin regulates spatially polarized signals through distinct p120ctn and beta-catenin-dependent signalling pathways. Nat Commun 2013; 4:1589; PMID:23481397; http://dx.doi.org/10.1038/ncomms2560
- Wong MK, Gotlieb AI. The reorganization of microfilaments, centrosomes, and microtubules during in vitro small wound reendothelialization. J Cell Biol 1988; 107:1777-83; PMID:3182937; http://dx.doi.org/10.1083/jcb.107.5.1777
- Desai RA, Gao L, Raghavan S, Liu WF, Chen CS. Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci 2009; 122:905-11; PMID:19258396; http://dx.doi.org/10.1242/jcs.028183
- Mishra R, Gupta SK, Meiri KF, Fong M, Thostrup P, Juncker D, Mani S. GAP-43 is key to mitotic spindle control and centrosome-based polarization in neurons. Cell Cycle 2008; 7:348-57; PMID:18235238; http://dx.doi.org/10.4161/cc.7.3.5235
- Paulson AF, Prasad MS, Thuringer AH, Manzerra P. Regulation of cadherin expression in nervous system development. Cell Adh Mig 2014; 8:19-28; PMID:24526207; http://dx.doi.org/10.4161/cam.27839
- Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol 2003; 253:309-23; PMID:12645933; http://dx.doi.org/10.1016/S0012-1606(02)00016-7
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron 2010; 67:588-602; PMID:20797536; http://dx.doi.org/10.1016/j.neuron.2010.07.007
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron 2007; 54:771-85; PMID:17553425; http://dx.doi.org/10.1016/j.neuron.2007.05.013
- Morita H, Nandadasa S, Yamamoto TS, Terasaka-Iioka C, Wylie C, Ueno N. Nectin-2 and N-cadherin interact through extracellular domains and induce apical accumulation of F-actin in apical constriction of Xenopus neural tube morphogenesis. Development 2010; 137:1315-25; PMID:20332149; http://dx.doi.org/10.1242/dev.043190
- Biswas S, Emond MR, Jontes JD. Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. The J Cell Biol 2010; 191:1029-41; PMID:21115806; http://dx.doi.org/10.1083/jcb.201007008
- Nakao S, Platek A, Hirano S, Takeichi M. Contact-dependent promotion of cell migration by the OL-protocadherin-Nap1 interaction. The J Cell Biol 2008; 182:395-410; PMID:18644894; http://dx.doi.org/10.1083/jcb.200802069
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 1999; 274:36734-40; PMID:10593980; http://dx.doi.org/10.1074/jbc.274.51.36734
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 2005; 6:622-34; PMID:16025097; http://dx.doi.org/10.1038/nrm1699
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J 2005; 24:742-52; PMID:15692570; http://dx.doi.org/10.1038/sj.emboj.7600548
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 1986; 320:447-9; PMID:3515198; http://dx.doi.org/10.1038/320447a0
- Redies C, Hertel N, Hubner CA. Cadherins and neuropsychiatric disorders. Brain Res 2012; 1470:130-44; PMID:22765916; http://dx.doi.org/10.1016/j.brainres.2012.06.020
- Doherty P, Walsh FS. The contrasting roles of N-CAM and N-cadherin as neurite outgrowth-promoting molecules. J Cell Sci Supplement 1991; 15:13-21; PMID:1824104; http://dx.doi.org/10.1242/jcs.1991.Supplement_15.3
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron 1996; 17:837-48; PMID:8938117; http://dx.doi.org/10.1016/S0896-6273(00)80216-0
- Bard L, Boscher C, Lambert M, Mege RM, Choquet D, Thoumine O. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci 2008; 28:5879-90; PMID:18524892; http://dx.doi.org/10.1523/JNEUROSCI.5331-07.2008
- Pollarolo G, Schulz JG, Munck S, Dotti CG. Cytokinesis remnants define first neuronal asymmetry in vivo. Nat Neurosci 2011; 14:1525-33; PMID:22081156; http://dx.doi.org/10.1038/nn.2976
- Gartner A, Fornasiero EF, Dotti CG. N-cadherin: a new player in neuronal polarity. Cell Cycle 2012; 11:2223-4; PMID:22659798; http://dx.doi.org/10.4161/cc.20797
- Gärtner A, Fornasiero EF, Valtorta F, Dotti CG. Distinct temporal hierarchies in membrane and cytoskeleton dynamics precede the morphological polarization of developing neurons. J Cell Sci 2014; 127:4409-19; PMID:25128563
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol 2007; 304:22-33; PMID:17222817; http://dx.doi.org/10.1016/j.ydbio.2006.12.014
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. Embo J 2004; 23:2314-24; PMID:15141162; http://dx.doi.org/10.1038/sj.emboj.7600223
- Tabata H, Nakajima K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ 2008; 50:507-11; PMID:18482404; http://dx.doi.org/10.1111/j.1440-169X.2008.01043.x
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 2011; 69:482-97; PMID:21315259; http://dx.doi.org/10.1016/j.neuron.2011.01.003
- Jossin Y, Cooper JA. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci 2011; 14:697-703; PMID:21516100; http://dx.doi.org/10.1038/nn.2816
- Jossin Y. Polarization of migrating cortical neurons by Rap1 and N-cadherin:revisiting the model for the Reelin signaling pathway. Small GTPases 2011; 2:322-8; PMID:22545231; http://dx.doi.org/10.4161/sgtp.18283
- Luccardini C, Hennekinne L, Viou L, Yanagida M, Murakami F, Kessaris N, Ma X, Adelstein RS, Mege RM, Metin C. N-cadherin sustains motility and polarity of future cortical interneurons during tangential migration. J Neurosci 2013; 33:18149-60; PMID:24227724
- Rieger S, Senghaas N, Walch A, Koster RW. Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol 2009; 7:e1000240; PMID:19901980; http://dx.doi.org/10.1371/journal.pbio.1000240
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Ann Rev Neurosci 2009; 32:347-81; PMID:19400726; http://dx.doi.org/10.1146/annurev.neuro.31.060407.125536
- Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci 2013; 116:409-32; PMID:23481205; http://dx.doi.org/10.1016/B978-0-12-394311-8.00018-2
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012; 149:1192-205; PMID:22682243; http://dx.doi.org/10.1016/j.cell.2012.05.012
