Abstract
Molecular oscillation of the circadian clock is based on E-box-mediated transcriptional feedback loop formed with clock genes and their encoding products, clock proteins. The clock proteins are regulated by post-translational modifications such as phosphorylation. We investigated the effects of a series of kinase inhibitors on gene expression rhythms in Rat-1 fibroblasts. The period of the cellular circadian rhythm in culture was lengthened by treatment with SB203580 (p38 MAPK inhibitor), SP600125 (JNK inhibitor), IC261 (CKI inhibitor) and Roscovitine (CDK inhibitor). On the other hand, the period was shortened by SB216763 (GSK-3 inhibitor) or KN93 (CaMKII inhibitor) treatment. Application of 20 μM KN93 completely abolished the rhythmic gene expression. The activity of CaMKII exhibited circadian variation in a phase close to the E-box-mediated transcriptional rhythms. In vitro kinase assay revealed that CaMKII directly phosphorylates N-terminal and Ser/Pro-rich domains of CLOCK, an activator of E-box-mediated transcription. These results indicate a phosphorylation-dependent tuning of the period length by a regulatory network of multiple kinases and reveal an essential role of CaMKII in the cellular oscillation mechanism.
Introduction
Many physiological and behavioral processes are under the control of the circadian clock.Citation1 In mammals, the circadian clock system is organized in a hierarchy of multiple layers of oscillators. A master pacemaker in the suprachiasmatic nucleus (SCN) of hypothalamus orchestrates an array of slave oscillators existing in peripheral tissues via humoral factors and neuronal controls.Citation2 The cell-autonomous molecular oscillation is based on the transcriptional feedback loop driven by the clock genes and their products in both the SCN and peripheral tissues. Circadian locomotor output cycles kaput (CLOCK) and Brain and Muscle Arnt-like protein-1 (BMAL1) both encode bHLH-PAS containing transcriptional factors, which form a heterodimer to activate transcription of Period (Per) and Cryptochrome (Cry) genes through a CACGTG E-box cis-element. In turn, translated and nuclear accumulated PER and CRY proteins interfere with CLOCK-BMAL1-dependent transcriptional activation, thereby repressing expression of their own genes. Disruption of these circadian regulators abolishes cellular gene expression rhythms, indicating their essential roles in the oscillation mechanism.
The period length of the transcriptional feedback loop is finely tuned by post-translational modifications of clock proteins, such as phosphorylation. Inhibition of casein kinase (CK)Iϵ and δ or c-Jun N-terminal kinase (JNK) results in lengthening of the period of the gene expression rhythms in mammalian cells.Citation3-10 In chick pineal cells, SB203580, an inhibitor of p38 mitogen-activated protein kinase (p38 MAPK), lengthens the period of the circadian rhythm of the melatonin release.Citation11 SB202190, another inhibitor of p38 MAPK, lengthens the period of the gene expression rhythms in cultured human cells.Citation10 Inhibitors of cyclin-dependent kinase (CDK) such as Roscovitine lengthen ocular circadian rhythm in Bulla and Aplysia and the gene expression rhythms in cultured human cells.Citation10,12,13 Inhibition of glycogen synthase kinase (GSK)-3β or dual-specificity tyrosine phosphorylation-regulated kinase (DYRK)1A results in shortening of the period length of the gene expression rhythms in mammalian cells.Citation10,14
Here we investigated the effects of a series of protein kinase inhibitors on bioluminescence rhythms from Bmal1-luciferase reporter in Rat-1 fibroblasts. The inhibitors examined in this study include those already reported in similar and/or other systems cited above.
Results
Screening of kinase inhibitors affecting molecular clock
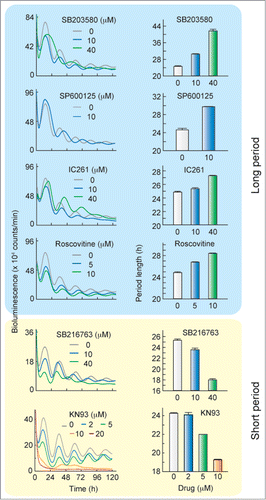
We investigated the effects of a series of kinase inhibitors on the cellular circadian rhythm by using Rat-1 fibroblasts expressing a Bmal1-luciferase reporter.Citation15,16 No significant change in the period length was observed in the presence of U0126 [mitogen-activated protein kinase kinase (MEK) inhibitor], Gö6983 [protein kinase C (PKC) inhibitor], TBBt [casein kinase II (CKII) inhibitor], KT5720 [protein kinase A (PKA) inhibitor] or KT5823 [protein kinase G (PKG) inhibitor] (). Inhibitors of phosphoinositide 3-kinase (PI3K), wortmannin and LY294002, had no significant effect or only marginal effects on the period length of the cellular rhythms (). The period of the rhythm was lengthened by treatment with SB203580 (p38 MAPK inhibitor), SP600125 (JNK inhibitor), IC261 (CKI inhibitor) or Roscovitine (CDK2/7/9 inhibitor] ( and ). On the other hand, the period length was shortened by SB216763 (GSK-3α/β inhibitor) or KN93 [Ca2+/calmodulin-dependent protein kinase (CaMKII) inhibitor]. These results demonstrate that the period length of the cellular clock is finely tuned by a network of the phosphorylation signaling mediated by multiple protein kinases. We found that the cellular oscillation was severely dampened in the presence of 20 μM KN93 (), and knock-down of CaMKIIγ and δ, 2 ubiquitous CaMKII isoforms, abolished the cellular rhythm in cultured cells.Citation16 These results demonstrate that CaMKII activity plays an essential role for robust oscillation of the cellular clock in addition to the regulatory role for the period length.
Table 1. Effects of kinase inhibitors on period length of Rat-1 cellular rhythm in culture
Figure 1. Period of the cellular clock is affected by treatment with SB203580, SP600125, IC261, Roscovitine, SB216763 or KN93. After treatment with 0.1 μM dexamethasone for 2 h, Rat-1-Bmal1-luc cells were transferred to the fresh medium containing 0.1 % DMSO with or without the kinase inhibitor. Left panels show representative raw results and right panels show mean period length with SEM from 4 independent experiments. The period length in the presence of 20 μM KN93 was not determined due to disruption of the bioluminescence rhythm.
CaMKII as an essential component of oscillation system
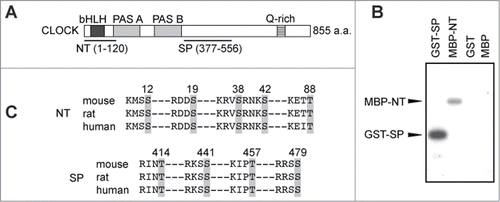
CaMKII activity in the SCN exhibits a circadian variation in a phase close to that of the E-box-dependent transcription rhythm.Citation1,17 The circadian variation of the CaMKII activity was also observed in the peripheral tissue, such as the lung (). Recently, we have shown that CaMKII stimulates expression of E-box-regulated genes by enhancing the heterodimerization of CLOCK and BMAL1 and that overexpression of CaMKII in HEK293 cells increases the phosphorylation level of CLOCK.Citation16 Here we used 2 partial domains of CLOCK protein termed as N-terminal (NT) and Ser/Pro-rich (SP) domains for in vitro CaMKII phosphorylation assay (). A constitutive active catalytic domain of CaMKII, 30K-CaMKII, phosphorylated GST-SP, a fusion protein of SP domain with glutathione S-transferase (GST) and MBP-NT, a fusion protein of NT domain with maltose-binding protein (MBP) (). On the other hand, no significant phosphorylation was detected with GST or MBP alone. These results indicate that CaMKII directly phosphorylates the SP and NT domains of CLOCK. It is possible that CaMKII-mediated phosphorylation of these domains is important for the heterodimerization of CLOCK with BMAL1 and for activation of the E-box-dependent gene expression.
Figure 2. Circadian activation of CaMKII in phase with E-box-regulated gene expression rhythm. Mice were entrained to 12-h light/12-h dark cycles, and the lung was isolated from mice sacrificed every 4-hours on the first day under the constant dark condition. The samples were subjected to immunoblotting (A) or RT-PCR analysis (B). (A) Circadian profile of the phosphorylation (activation) levels of CaMKII. The activation levels of CaMKII were estimated by using an antibody recognizing phosphorylated T286 on CaMKII (Sigma-Aldrich), which represents its activated form. Top and middle panels show raw data for phospho-CaMKII and β-actin, respectively, and the band intensities of the former were quantified from 6 independent experiments (bottom panel). Data are mean with SEM, and the significant change is observed (P < 0.05, ANOVA). (B) Circadian changes in Per1 and Dbp mRNA levels. The mRNA signals obtained by RT-PCR analysis were normalized to Gapdh mRNA. Data are mean with SEM from 4 independent experiments.
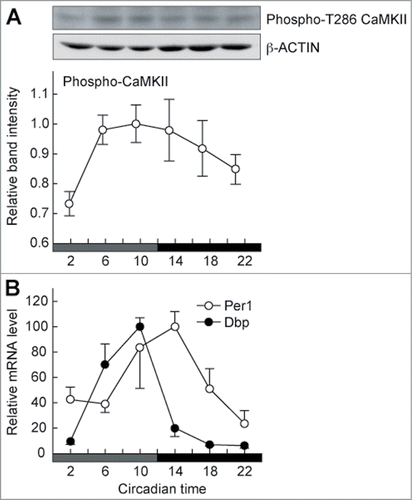
Figure 3. N-terminal region and Ser/Pro-rich region of CLOCK is phosphorylated by CaMKII. (A) Schematic drawing of the structure of mouse CLOCK protein. The N-terminal (NT) and Ser/Pro-rich (SP) region of CLOCK protein were subjected to the in vitro CaMKII phosphorylation assay. (B) In vitro CaMKII phosphorylation assay. GST-SP, MBP-NT, GST or MBP was used as a substrate protein for the in vitro CaMKII phosphorylation assay. GST-SP and MBP-NT were phosphorylated by 30K-CaMKII, whereas no significant phosphorylation was detected with GST or MBP. (C) Consensus CaMKII phosphorylation sequences (R/KXXS/T) in NT and SP region of CLOCK. The consensus sequences of mouse CLOCK were aligned with the corresponding regions of rat and human CLOCK. Gray areas indicate potential phosphorylation sites.
Conclusion
A cell-based phenotype screening of small molecule compounds is a very useful approach to identify modifying enzymes involved in the cellular clockwork.Citation7,10,15,18,19 The present study revealed that the period of the cellular clock was lengthened by SB203580, SP600125, IC261 and Roscovitine, consistent with the previous studies.Citation3-13 On the other hand, the period was shortened by SB216763 or KN93. We recently reported the roles of CaMKII in regulation of the circadian clock at multiple levels.Citation16 In the cellular level, CaMKII mediates Ca2+-dependent regulation of the transcriptional feedback loop by activating E-box-dependent gene expression. CaMKII directly phosphorylates CLOCK (), and the NT or SP domain of CLOCK contains 5 or 4 CaMKII consensus sequences, R/KXXS/T,Citation20 respectively (). In the SCN, CaMKII activity is essential for synchronization of individual neuronal rhythms and for the synchronized oscillation between left and right SCN nuclei.Citation16 In contrast to the effect of KN93 on the period length in the cultured cells (), mice carrying a kinase-dead mutation in CaMKIIα (K42R) showed prolonged period length in wheel running rhythms.Citation16 Because the previous study demonstrated that inhibition of neuronal coupling among the SCN neurons resulted in prolongation of the period in behavioral rhythms,Citation21,22 it is possible that disruption of the neuronal coupling by the CaMKII mutation might have affected strongly the period length of the behavioral rhythms. Further behavioral analysis of the CaMKIIα mutant mice revealed that the kinase activity is important not only for the robust wheel running rhythm but also for the coupling between the morning and evening activity rhythms. In this way, our cell-based kinase inhibitor screening revealed CaMKII as an important mediator in the communication between the morning and evening oscillators in the behavioral rhythms.Citation16 Such behavioral phenotypes were quite unique, and further analysis of the mutant mice will provide a novel insight into the circadian regulation of the behavioral activities.
Materials & Methods
Real-time monitoring of circadian rhythms in cultured cells
Real-time monitoring of the circadian gene expression was performed using Rat-1 cells expressing a luciferase reporter under the control of the upstream region of Bmal1 gene.Citation15,16 Rat-1 cells were treated with 0.1 μM dexamethasone for 2 h for the rhythm synchronization, and the bioluminescence signals from the cells were continually recorded at 37°C under air with dish-type bioluminescence detector, Kronos (ATTO, AB-2500) or LumiCycle (Actimetrics).
In vitro CaMKII phosphorylation assay
The in vitro kinase assay was carried out at 30°C in a reaction mixture (10 μl) consisting of 1 mM dithiothreitol, 100 μM [γ-32P]ATP, 10 ng 30K-CaMKII and 100 ng substrate protein.Citation23 The substrate proteins, GST-SP, MBP-NT, GST or MBP (each 100 ng) were prepared as described previously.Citation24 After incubation for 30 min, the reaction was stopped by the addition of 10 μl of 2 × SDS sample buffer. Phosphorylated proteins were resolved by SDS-PAGE and detected by autoradiography.
Animal Experiments
The animal experiments were conducted in accordance with the guidelines of the University of Tokyo. The mice (C57BL/6 background, male, 10-week-old) were housed individually at 23°C in cages with food and water available ad libitum. Immunoblotting and RT-PCR analyses were performed as described previously.Citation15,16,24
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from JSPS and MEXT, Japan. N. K. and Y. S. were supported by JSPS Research Fellowships for Young Scientists.
References
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell 2002; 111:919-22; PMID:12507418; http://dx.doi.org/10.1016/S0092-8674(02)01225-4
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 2010; 72:551-77; PMID:20148688; http://dx.doi.org/10.1146/annurev-physiol-021909-135919
- Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol 2005; 25:2795-807; PMID:15767683; http://dx.doi.org/10.1128/MCB.25.7.2795-2807.2005
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sládek M, Semikhodskii AS, Glossop NR, Piggins HD, et al. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 2008; 58:78-88; PMID:18400165; http://dx.doi.org/10.1016/j.neuron.2008.01.019
- Walton KM, Fisher K, Rubitski D, Marconi M, Meng QJ, Sládek M, Adams J, Bass M, Chandrasekaran R, Butler T, et al. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J Pharmacol Exp Ther 2009; 330:430-9; PMID:19458106; http://dx.doi.org/10.1124/jpet.109.151415
- Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto KH, Kiuchi R, Ishida M, Ukai-Tadenuma M, Minami Y, et al. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci U S A 2009; 106:15744-9; PMID:19805222; http://dx.doi.org/10.1073/pnas.0908733106
- Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, Ye ZY, Pan HL, Takahashi JS. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A 2012; 109:101-6; PMID:22184224; http://dx.doi.org/10.1073/pnas.1118034108
- Chansard M, Molyneux P, Nomura K, Harrington ME, Fukuhara C. c-Jun N-terminal kinase inhibitor SP600125 modulates the period of mammalian circadian rhythms. Neuroscience 2007; 145:812-23; PMID:17270352; http://dx.doi.org/10.1016/j.neuroscience.2006.12.037
- Yoshitane H, Honma S, Imamura K, Nakajima H, Nishide SY, Ono D, Kiyota H, Shinozaki N, Matsuki H, Wada N, et al. JNK regulates the photic response of the mammalian circadian clock. EMBO Rep 2012; 13:455-61; PMID:22441692; http://dx.doi.org/10.1038/embor.2012.37
- Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci U S A 2008; 105:20746-51; PMID:19104043; http://dx.doi.org/10.1073/pnas.0811410106
- Hayashi Y, Sanada K, Hirota T, Shimizu F, Fukada Y. p38 mitogen-activated protein kinase regulates oscillation of chick pineal circadian clock. J Biol Chem 2003; 278:25166-71; PMID:12719413; http://dx.doi.org/10.1074/jbc.M212726200
- Krucher NA, Meijer L, Roberts MH. The cyclin-dependent kinase (cdk) inhibitors, olomoucine and roscovitine, alter the expression of a molluscan circadian pacemaker. Cell Mol Neurobiol 1997; 17:495-507; PMID:9353591; http://dx.doi.org/10.1023/A:1026358821640
- Sankrithi N, Eskin A. Effects of cyclin-dependent kinase inhibitors on transcription and ocular circadian rhythm of Aplysia. J Neurochem 1999; 72:605-13; PMID:9930732; http://dx.doi.org/10.1046/j.1471-4159.1999.0720605.x
- Kurabayashi N, Hirota T, Sakai M, Sanada K, Fukada Y. DYRK1A and glycogen synthase kinase 3beta, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol Cell Biol 2010; 30:1757-68; PMID:20123978; http://dx.doi.org/10.1128/MCB.01047-09
- Kon N, Hirota T, Kawamoto T, Kato Y, Tsubota T, Fukada Y. Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat Cell Biol 2008; 10:1463-9; PMID:19029909; http://dx.doi.org/10.1038/ncb1806
- Kon N, Yoshikawa T, Honma S, Yamagata Y, Yoshitane H, Shimizu K, Sugiyama Y, Hara C, Kameshita I, Honma K, et al. CaMKII is essential for the cellular clock and coupling between morning and evening behavioral rhythms. Genes Dev 2014; 28:1101-10; PMID:24831701; http://dx.doi.org/10.1101/gad.237511.114
- Agostino PV, Ferreyra GA, Murad AD, Watanabe Y, Golombek DA. Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem Int 2004; 44:617-25; PMID:15016477; http://dx.doi.org/10.1016/j.neuint.2003.09.005
- Yagita K, Yamanaka I, Koinuma S, Shigeyoshi Y, Uchiyama Y. Mini screening of kinase inhibitors affecting period-length of mammalian cellular circadian clock. Acta Histochem Cytochem 2009; 42:89-93; PMID:19617956; http://dx.doi.org/10.1267/ahc.09015
- Jones KA, Hatori M, Mure LS, Bramley JR, Artymyshyn R, Hong SP, Marzabadi M, Zhong H, Sprouse J, Zhu Q, et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol 2013; 10:630-5; PMID:23974117; http://dx.doi.org/10.1038/nchembio.1333
- White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem 1998; 273:3166-72; PMID:9452427; http://dx.doi.org/10.1074/jbc.273.6.3166
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MN. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 2008; 320:949-53; PMID:18487196; http://dx.doi.org/10.1126/science.1152506
- Doi M, Ishida A, Miyake A, Sato M, Komatsu R, Yamazaki F, Kimura I, Tsuchiya S, Kori H, Seo K, et al. Circadian regulation of intracellular G-protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat Commun 2011; 2:327; PMID:21610730; http://dx.doi.org/10.1038/ncomms1316
- Sugiyama Y, Ishida A, Sueyoshi N, Kameshita I. Tyrosine kinase activity of a Ca2+/calmodulin-dependent protein kinase II catalytic fragment. Biochem Biophys Res Commun 2008; 377:648-52; PMID:18930024; http://dx.doi.org/10.1016/j.bbrc.2008.10.028
- Yoshitane H, Takao T, Satomi Y, Du NH, Okano T, Fukada Y. Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription. Mol Cell Biol 2009; 29:3675-86; PMID:19414601; http://dx.doi.org/10.1128/MCB.01864-08
