Abstract
One major gap in adipocyte biology has been a lack of understanding of the developmental origins of the different visceral white adipose tissue (WAT) depots and subcutaneous WAT. In a recent study we showed that most visceral WAT but no subcutaneous WAT arises from cells expressing the Wilms’ tumor 1 (Wt1) gene late in mouse gestation.Citation1 Wt1 continues to be expressed in visceral WAT progenitors into adult life. We also showed that visceral WAT is lined by a mesothelium and provided evidence that this structure is the source of adipocytes. Our study also adds to the growing body of evidence that there is heterogeneity in the visceral progenitors, such that there are Wt1-expressing and non-expressing subsets, the relative proportions of which vary between depots. This raises the enticing prospect that the adipocytes arising from these progenitor subsets may have different properties and our preliminary data support this notion. Finally, evidence from our study and one from Spiegelman's groupCitation2 suggests that Wt1 is not just a marker but regulates visceral WAT identity and the progenitor population. We discuss the implications of this work and some of the questions and future directions that arise from it.
Adipose tissues are the body's natural sites for storing excessive calories. They are composed of multiple types of cells including the mature adipocytes which make up the bulk of the tissues as well as the SVF (stromal vascular fractions, including the endothelial cells, immune cells, preadipocytes, and adipose progenitor/stem cells). Fat can be divided into several types based on the color and location. White adipose tissues (WAT) are characterized by the presence of adipocytes with large mono-locular lipid droplets while adipocytes in the brown adipose tissues (BAT) have smaller and multi-locular lipid droplets. BATs are darker in color due to the richness in blood vessels and mitochondria content. Beige/brite adipose tissue is the newly identified category of fat.Citation3-5 It is believed that progenitors of the beige adipose tissues normally reside in the WATs and the ‘browning’ process is switched on by exposure to cold. Genes and pathways that are involved in the browning process have been identified by studies reported in several elegant papers.Citation6-9 Secreted and systemic factors that have been shown to induce the browning process are summarized in several recent reviews.Citation10-12
Identifying players that are involved in adipogenesis and the browning process has provided new clues to battle obesity.Citation13-17 Most of the adipose tissues in our body are WATs which can be further divided into the subcutaneous and the visceral fat. Individuals with central obesity,which is associated with higher risk of obesity-related diseases including type II diabetes, cardiovascular diseases and cancer, tend to have larger amounts of visceral adipose tissue (the so called ‘bad fat’). On the other hand, individuals with larger amounts of subcutaneous fat tend to have better metabolic profiles and lower risks of obesity-related diseases.Citation18 Fat pad transplantation experiment in mice suggested that the ‘good’ versus ‘bad’ properties are intrinsic to the fat pads, and the location makes a relatively small contribution.Citation19
Despite the knowledge that the fat pads differ in their cellular compositionCitation20-22 and the ability to proliferate/expand in response to environmental stimulations such as high fat diet,Citation23 their developmental origin has remained unclear. Fat is generally regarded as coming from the mesoderm except for the depots that are in the cranial facial area which come from the neural crestCitation24 (ectodermal origin). In mice, Seale et al (2008) demonstrated that the myf5+ cells in the paraxial mesoderm give rise to the BAT and not WAT.Citation7 A recent paper by Sanchez-Gurmaches et al showed that myf5+ cells can also contribute to adipocytes in retroperitoneal but not epididymal WAT.Citation25 Fat depots also differ in the timing at which they first appear in the body. In the murine system, BAT is visible as early as from E14.5Citation26 while subcutaneous/inguinal fat develops from E14-E18.Citation27
The pre-adipocytes isolated from the SVF of fat show differences in their gene expression between visceral depots as well as between visceral and subcutaneous fat.Citation28 However, Tchkonia et al demonstrated that pre-adipocytes from mesenteric fat had an expression profile closer to that of subcutaneous than omental preadipocytes using adult human fat tissue obtained during intra-abdominal surgery.Citation21 In the study carried out by Yamamoto et al where adult mouse tissue was analyzed, the expression of some developmental genes (e.g. En1) was high in subcutaneous and perirenal WAT, but low in epididymal and mesenteric WATs.Citation22 From the gene expression studies, it was not possible to conclude if the subcutaneous or visceral WATs have a common origin. Our work showed that, despite both being WATs, the visceral and subcutaneous adipose tissues have different developmental origins. Our study also provided evidence that the mesothelium is a novel source for the visceral adipose tissues,Citation1 thus tracing their origin to the lateral plate mesoderm. Identifying different origins and progenitors for visceral vs. subcutaneous WAT could lead to approaches that selectively manipulate these populations for therapy.
Wt1 Marks Adipocyte Progenitors in All Visceral Fat Depots
Our initial interest in studying adipose tissue was to understand the reason behind the rapid fat loss when we deleted Wt1 (Wilms Tumor protein gene) ubiquitously in adult mice.Citation29 Adult mice in which Wt1 was deleted also showed a rapid bone loss. We showed that Wt1 expression was detected in all visceral fat depots but not in the subcutaneous WAT or the BAT. Bone and adipose tissues share the same origin, ‘mesenchymal stem cells’ which led us to investigate if the Wt1-expressing adipose cells had any characteristics of progenitors. All Wt1+ cells were in the SVF and not in the floating mature adipocytes. Great advances in defining and identifying stem cells in adipose tissues have been made by showing that transplanted FACS isolated cells (expressing combinations of surface markers) were able to form functional adipose depots.Citation30 Rodeheffer et al showed that the Lin-CD34+CD29+Sca1+CD24+ cells (hereafter called CD24+) are able to form fat pads when transplanted in lipodystrophic mice, and referred to this population of cells as the adipose stem cells.Citation31 Later work from Rodeheffer's group demonstrated that the Lin-CD34+CD29+Sca1+CD24- cells (CD24-) are preadipocytes and the CD24+ population give rise to the CD24- population.Citation31 Using this panel of surface markers, we showed that the majority of Wt1+ cells in the SVF were in the CD24- population. Some Wt1+ cells were also found in the CD24+ population. The proportion of the CD24+ or CD24- populations that were expressing Wt1 differed between fat pads. Omental and heart fat had the highest percentage of cells that were expressing Wt1.Citation1
Lineage Tracing Studies Showed that Wt1+ Progenitors Gave Rise to Adipocytes in Visceral Fat Depots
To test if the Wt1+ adipose progenitors can give rise to adipocytes, we performed lineage tracing studies using the tamoxifen inducible Wt1-CreERT2 transgenic line, crossed with the mTmG reporter line. As mentioned by Berry et al.,Citation32 we also found that the mTmG reporter line is a valuable tool for studying adipocytes. Most of the volume of any mature adipocyte is occupied by lipid droplet(s) and the nuclei are pushed to the side. The membranous nature of the fluorescent signal in the mTmG reporter line provides a useful method of marking adipocytes. In our study, labeled adipocytes were observed in the visceral adipose depots (but not subcutaneous) when tamoxifen was administered in adult mice proving that at least some adipocytes arise from Wt1 progenitors postnatally. We were interested in knowing the developmental origin of WATs. Therefore we then administered tamoxifen in pregnant females at E14.5 and analyzed the adipose tissues in the offspring after 1.2 y. A high percentage of adipocytes was positive suggesting these adipocytes came from cells that were Wt1 positive at E14.5. We did not observe any positive signal in the subcutaneous WAT or the BAT in this model. It is suggested that tamoxifen can be retained in the system for up to 48 hours when administered during embryonic stages.Citation33 We concluded that these adipocytes must have come from cells that expressed Wt1 between E14.5-E16.5.
The Mesothelium is a Source of Visceral Fat Progenitors
We were fascinated by the physical attachment of visceral adipose depots to the organs. All visceral organs (visceral mesothelium) and the entire body cavity (parietal mesothelium) are covered by a layer of cells called the mesothelium.Citation1 Mesothelia have been shown to contribute to the smooth muscle cells, endothelial cells, and interstitial cells in the heart, liver, lung, and gut during development.Citation34-40 In adults, the main function of mesothelia is to provide lubrication allowing movement of the visceral organs within the body cavity. They are also a known source of progenitor cells involved in scarring and neovascularization during injury.Citation41 To our surprise, we observed that visceral fat depots were also covered by a layer of mesothelium.Citation1
Wt1 is a well-known marker for mesothelia. The timing at which the mesothelia first appear and the mechanisms of mesothelia formation differ for each organ. In the E9.5 heart in mice, cells in the proepicardium migrate and form a sheet of cells which then cover the surface of the heart forming the epicardium (mesothelium of the heartCitation42). In the intestine and lung, recent work by Bader and colleagues showed that the progenitors of mesothelium that covers the lung and gut reside in the respiratory and alimentary tracts (these lack an equivalent to the proepicardium structure seen for epicardium formation).Citation43
We hypothesized that mesothelia might be a source of visceral fat and this would explain the reason why only visceral depots were positive for adipocytes that came from Wt1-expressing cells. To test this, we cultured the epididymal appendage of the newborn pups from the tamoxifen-inducible Wt1-lineage tracing model. The epididymal appendage from the newborn pups is a thin translucent sheet of cells which will later form the epididymal fat. Cells with lipid droplets in the epididymal fat are not visible till postnatal day 4–7.Citation44 Whole mount staining of the epididymal appendage showed that it was covered by mesothelia. We cultured this sheet in matrigel supplemented with adipogenesis media and 4-OH tamoxifen and demonstrated that Wt1-lineaged cells in this sheet were capable of making adipocytes. In addition, we showed that Wt1+ cells in this sheet expressed high levels of mesothelial markers such as mesothelin and UPK3B. We also performed a short lineage tracing experiment by dosing the female with tamoxifen at E14.5 and analyzed the embryos at E16.5 and showed that mesothelia that covered the surface of visceral organs were labeled.
We also determined whether the mesothelial cells expressed any of the adipose progenitor markers. The panel of adipose stem cell markers is based on work characterized in adipose tissues and had not previously been functionally tested in other tissues. Our result showed that most adult and postnatal day 1 mesothelial cells were in the Lin-CD31-CD34+CD29+Sca1+CD24- populations. Mesothelial cells from earlier developmental stages showed a similar but not identical pattern of expression. At E14.5, most mesothelial cells were in the Lin-CD31-CD34+CD29+Sca1- but CD24+ population. The mesothelial cells lost CD24 expression and gained expression of Sca1 rapidly as development progressed, a similar scenario to that observed for fat progenitors in the SVFCitation31 This demonstrated that the gene expression pattern of mesothelia was not in a fixed state but it was dynamic and underwent rapid changes. Analyzing the exact genetic blueprint of mesothelia at specific developmental stages, ages, variation between organs, and their functional outputs is likely to give new insights into pathways involved in adipogenesis and how these differ from other mesothelia-associated tissues.
Although we have provided multiple evidence suggesting mesothelia could be a potential visceral WAT source including showing that 1) visceral WAT has a mesothelial layer and Wt1 is one of the most commonly used markers for studying mesothelia, 2) Wt1+ cells in the epididymal appendage expressed high levels of mesothelia-enriched genes, 3) only mesothelia were labeled in the short tamoxifen pulsing experiment (E14.5-E16.5), and 4) mesothelial cells expressed adipose progenitor markers, we would still like to strengthen this result by using a mesothelial-specific cre (e.g., the mesothelin-CreCitation41) for cell fate analysis in vivo. However, in support of our hypothesis, a recent study using lineage tracing showed that embryonic epicardial progenitors were able to contribute to epicardial fat in postnatal 8 weeks old mice. This was achieved by injecting an adenovirus that expresses mesothelin (Msln) Cre in the E12.5 Rosa26mTmG/+ embryonic heart.Citation45
Adipose tissue is often seen to be characterized by ill-defined boundaries. However, we noticed that visceral adipose tissues can still be separated into discrete depots even in obese models in rodents. We propose that the mesothelia covering the surface of visceral adipose depots help to keep the depots in shape as well as to dictate where fat is formed. The adipose mesothelia may perform the same functions as mesothelia have in other organs (e.g. paracrine effects and producing mesenchymal progenitors through an epithelial to mesenchyme transition or more correctly a mesothelial to mesenchyme transition). Given this and the fact that each fat depot has a different composition of cells and this is reflected by their distinctive transcriptomic profiles, it is tempting to consider the depots as little ‘mini-organs’ with different ‘flavors’ and functions.Citation46
Heterogeneity of Visceral WAT Progenitors
Our lineage data and FACS analysis suggested that not all visceral WAT progenitors express Wt1 and the proportion of Wt1+ progenitors varies between depots. Preliminary data in our study also suggested that the adipocytes that arose from Wt1+ progenitors differed in their physical properties (size and number of droplet/cell) from the adipocytes that were derived from Wt1-negative progenitors. The chemical, transcriptomic, metabolomic properties, and turnover rate of the adipocytes arising from these 2 progenitors await to be revealed.
The turnover rate of adipocytes is not totally clear (estimates vary from 1–5%per day in miceCitation47 or a 10% turnover of adipocytes per year in humanCitation48). The fact that we saw adipocytes that were lineage traced from E14.5 after 1.2 y suggested that either the cells that were positive for Wt1 at E14.5 had the ability to self-renew or the adipocytes that were highlighted had very slow (or no) turnover, or both. Future studies determining the half-life and turnover rate of adipocytes are of great importance.
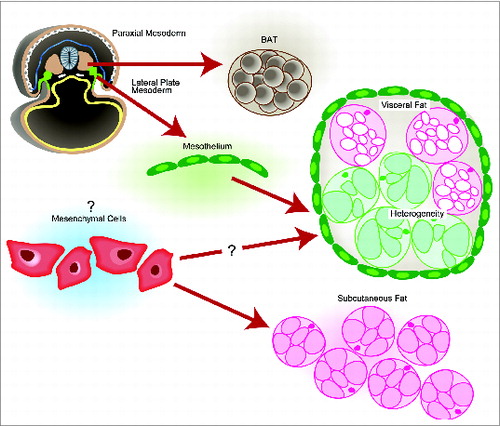
A cartoon illustration of the origin and heterogeneity of adipose tissue is summarized in . In an E8.5 mouse embryo, Wt1 is first expressed in the intermediate mesoderm and lateral plate mesoderm (highlighted in green). At E14.5, the Wt1-expressing mesothelia, derived from the lateral plate mesoderm, were shown to be one possible source of mature adipocytes in all visceral fat pads. The myf5-positive population of cells in the paraxial mesoderm have been shown to contribute to BAT as well as retroperitoneal WAT, adding to the heterogeneity of visceral WAT. The exact developmental origin of the subcutaneous WAT is not known although it is often to be described ambiguously as coming from ‘mesenchymal cells.’
Figure 1. A simplified cartoon placing our findings (Reference 1) into the context of pre-existing knowledge on the origins of the major murine adipose tissues. In an E8.5 mouse embryo, the Myf5+ cells in the paraxial mesoderm give rise to BAT. Mesothelial cells (express Wt1, indicated in green), which come from the lateral plate mesoderm, were able to contribute to adipocytes in all visceral fat pads (indicated in green). Like other visceral organs, visceral fat pads are also covered by mesothelium. The origin of subcutaneous WAT is not clear.
Wt1 was first associated with adipose tissue when we deleted Wt1 ubiquitously and the mutant mice had a fat loss phenotype.Citation29 The visceral only expression and lineage result provoked us to explore the developmental origin of fat. The next stage will be to investigate the function of Wt1 in regulating adipogenesis. We showed that Wt1 might be important for the growth of adipose progenitors. Deleting Wt1 in adipose depots led to a reduction of adipose progenitors in some depots.Citation1 Encouragingly, Spiegleman and colleagues showed that the thermogenic and inflammation pathways were switched on when Wt1 was deleted using an adiponectin-Cre.Citation2 In addition, in support of our mesothelial hypothesis, 2 genes that ranked top among the visceral progenitor markers in their paper are mesothelial genes (mesothelin and uroplakin). This brings Wt1 to the center stage of visceral adipogenesis. We have identified a population of adipose progenitors that were only expressed in the visceral adipose depots. This provides a useful tool in answering some key questions in this field including the heterogeneity of progenitors and adipocytes that are derived from them.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl, Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 2014; 16:367-75; PMID: 24609269; http://dx.doi.org/10.1038/ncb2922
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MK, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014; 156:304-16; http://dx.doi.org/10.1016/j.cell.2013.12.021
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G., Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Walden TB, Hansén IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. AM J Physiol Endocrinol Metab 2012; 302:E19-31; http://dx.doi.org/10.1152/ajpendo.00249.2011
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J., Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/10.1074/jbc.M109.053942
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012; 149:871-85; http://dx.doi.org/10.1016/j.cell.2012.02.066
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454:961-7; http://dx.doi.org/10.1038/nature07182
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454:1000-4; http://dx.doi.org/10.1038/nature07221
- Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol 2012; 14:1330-5; http://dx.doi.org/10.1038/ncb2612
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/10.1038/nm.3361
- Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab 2013; 17:638-43; PMID:23583169; http://dx.doi.org/10.1016/j.cmet.2013.02.020
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013; 27:234-50; PMID:23388824; http://dx.doi.org/10.1101/gad.211649.112
- Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Ivest 1995; 96:2914-23; http://dx.doi.org/JCI118363
- Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Diaz MB, Rozman J, Hrabe de Angelis M, Nüsing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 2010; 328:1158-61; http://dx.doi.org/10.1126/science.1186034
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011; 121:96-105; http://dx.doi.org/10.1172/JCI44271
- Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Muñoz-Martin M, Gómez-López G, Cañamero M, Mulero F, Pastor J, Martinez S, Romanos E, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab 2012; 15:382-94; http://dx.doi.org/10.1016/j.cmet.2012.02.001
- Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MK, Boström P, Mepani RJ, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 2012; 151:96-110; http://dx.doi.org/10.1016/j.cell.2012.08.034
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000; 21:697-738; http://dx.doi.org/10.1210/edrv.21.6.0415
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008; 7:410-20; PMID:18460332; http://dx.doi.org/10.1016/j.cmet.2008.04.004
- Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012: 61:1691-99; http://dx.doi.org/10.2337/db11-1753
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I., et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab 2007; 292:E298-307; PMID:16985259; http://dx.doi.org/10.1152/ajpendo.00202.2006
- Yamamoto Y, Gesta S, Lee KY, Tran TT, Saadatirad P, Kahn CR. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 2010; 18:872-8; PMID:20111017; http://dx.doi.org/10.1038/oby.2009.512
- Joe, AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009; 27:2563-70; http://dx.doi.org/10.1002/stem.190
- Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development 2007; 134:2283-92; http://dx.doi.org/10.1242.dev.002642
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from the Myf5 precursors. Cell Metab 2012; 16:348-62; PMID: 22940198; http://dx.doi.org/10.1016/jcmet.2012.08.003
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4:585-95; PMID:10549290; http://dx.doi.org/10.1016/S1097-2765(00)80209-9
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/10.1038/nm.3324
- Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 2006; 103:6676-81; PMID:16617105; http://dx.doi. org/10.1073/pnas.0601752103
- Chau YY, Brownstein D, Mjoseng H, Lee WC, Buza-VidasN, Nerlov C, Jacobsen SE, Perry P, Berry R, Thornburn A., et al. Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet 2011; 7:e1002404; PMID:22216009; http://dx.doi.org/10.1371/journal.pgen.1002404
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135:240-9; http://dx.doi.org/10.1016/j.cell.2008.09.036
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 2013; 15:302-8; PMID:23434825; http://dx.doi.org/10.1038/ncb2696
- Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab 2014; 19:8-20; PMID:24239569; http://dx.doi.org/10.1016/j.cmet.2013.10.003
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 2002; 244:305-18; PMID:11944939; http://dx.doi.org/10.1006/dbio.2002.0597
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A 2008; 105:16626-30; PMID:18922767; http://dx.doi.org/10.1073/pnas.0808649105
- Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 2011; 53:983-95; PMID:21294146; http://dx.doi.org/10.1002/hep.24119
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005; 132:5317-28; PMID:16284122; http://dx.doi.org/10.1242/dev.02141
- Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, Hall E, Reichmann J, Devenney PS, Hohenstein P., et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet 2010; 42:89-93; PMID:20023660; http://dx.doi.org/10.1038/ng.494
- Cano E, Carmona R, Munoz-Chapuli R. Wt1-expressing progenitors contribute to multiple tissues in the developing lung. Am J Physiol Lung Cell Mol Physiol 2013; 305:L322-332; PMID:23812634; http://dx.doi.org/10.1152/ajplung.00424.2012
- Carmona R, Cano E, Mattiotti A, Gaztambide J, Munoz-Chapuli R. Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos. PLoS One 2013; 8:e55890; PMID:23418471; http://dx.doi.org/10.1371/journal.pone.0055890
- Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol 2012; 14:1251-60; PMID:23143399; http://dx.doi.org/10.1038/ncb2610
- Elmadbouh I, Chen Y, Louedec L, Silberman S, Pouzet B, Meilhac O, Michel JB. Mesothelial cell transplantation in the infarct scar induces neovascularization and improves heart function. Cardiovasc Res 2005; 68:307-17; PMID:15979058; http://dx.doi.org/10.1016/j.cardiores.2005.05.022
- Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec 1981; 201:157-68; PMID:7305017; http://dx.doi.org/10.1002/ar.1092010117
- Winters NI, Williams AM, Bader DM. Resident progenitors, not exogenous migratory cells, generate the majority of visceral mesothelium in organogenesis. Dev Biol 2014; 391:125-32; PMID:24746591; http://dx.doi.org/10.1016/j.ydbio.2014.04.003
- Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development 2011; 138:5027-37; PMID:22028034; http://dx.doi.org/10.1242/dev.067686
- Liu Q, Huang X, Oh JH, Lin RZ, Duan S, Yu Y, Yang R, Qiu J, Melero-Martin JM, Pu WT., et al. Epicardium-to-fat transition in injured heart. Cell Res 2014; 24:1367-9; PMID:25257468; http://dx.doi.org/10.1038/cr.2014.125
- Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev 2012; 8:55-66; PMID:21365256; http://dx.doi.org/10.1007/s12015-011-9242-x
- Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid cellular turnover in adipose tissue. PLoS One 2011; 6:e17637; PMID:21407813; http://dx.doi.org/10.1371/journal.pone.0017637
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T., et al. Dynamics of fat cell turnover in humans. Nature 2008; 453:783-7; PMID:18454136; http://dx.doi.org/10.1038/nature06902
