Abstract
Some evidence suggests that monoclonal antibodies (mAb) can induce an adaptive immune response against tumor cells (“vaccinal effect”). Recently, we have shown that an anti-CD137 mAb can enhance the “vaccinal effect” of an anti-tumor mAb (cetuximab), thereby transforming a passive, monoclonal, short-term immunotherapy into an active, polyclonal, long-lasting immune response.
Monoclonal antibodies (mAb) directed against tumor cells have become an important part of the therapeutic armamentarium of cancer. The mechanisms of their antitumor activity include direct induction of apoptosis, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).Citation1 Although somewhat controversial, several observations indicate that mAbs may also induce an adaptive immune response referred to as the “vaccinal effect”.Citation2 In preclinical models, it has been shown that mAbs can enhance cross-presentation and promote adaptive immune responses.Citation3-5 In patients, several studies have indicated that maximal responses to mAb therapy may take several months, potentially suggesting that short-term cytolytic mechanisms (apoptosis, CDC, ADCC) are not the only ones involved. Additionally, retreatment of relapse patients with some mAbs can increase the time to progression, possibly because the second course may reactivate an adaptive immune response. Finally, mAb therapy can induce anti-tumor T cells that can be found in patients.Citation6-8 Enhancing the “vaccinal effect” of mAbs appears desirable as it could transform a passive, short-term immunotherapy into an active, long-lasting immune response.
We have previously shown that a stimulatory mAb directed against CD137 enhances NK cell-mediated ADCC induced by mAbs directed against tumor cells.Citation9,10 Recently, we have shown that an anti-CD137 mAb can also enhance the “vaccinal effect” of an anti-tumor mAb.Citation8
CD137 (4-1BB) is a costimulatory receptor that belongs to the tumor necrosis factor receptor superfamily. It is expressed on a variety of immune cells following activation, including T cells, dendritic cells (DC) and NK cells. Using EGFR-expressing cell lines from head and neck (HN) and colorectal cancer (CRC), we first demonstrated that an agonistic anti-CD137 mAb enhanced cetuximab-induced ADCC,Citation8 as previously shown in lymphoma and breast cancer with rituximab and trastuzumab, respectively.Citation9,10 As expected, depletion of NK cells almost completely abrogated the therapeutic benefit of cetuximab and anti-CD137 combination in immunocompetent mouse models. However, depletion of CD8 T cells also abrogated the efficacy of the combination therapy, suggesting a role for the adaptive immune response. Additionally, mice previously cured with combination therapy and untreated mice that received adoptively transferred CD8+ splenocytes from cured animals were protected from tumor (re)-challenge, supporting the development of an immune memory. Interestingly, these mice were protected against EGFR-positive and negative tumors (TUBO-EGFR and TUBO), arguing for epitope spreading. To further investigate the mechanisms of action of the combination, we developed a 2-tumor model in which mice where challenged simultaneously with EGFR-positive and negative tumors (TUBO-EGFR and TUBO) at 2 different sites. Combination therapy induced regression of both tumors whereas cetuximab alone was efficient only in TUBO-EGFR tumors. Interestingly, we had previously shown in another 2-tumor model (HER2-positive and negative tumors) that the therapeutic efficacy of anti-tumor mAb (trastuzumab) and anti-CD137 mAb combination was confined to the specific antigen (HER2-positive tumors) in nude mice, which lack T cells. This result suggests that T cells are necessary for eradicating the cancer cells that are not recognized by the tumor-directed mAb.Citation10 In the current study, cetuximab treatment induced NK cell and dendritic cell (DC) infiltration in TUBO-EGFR but not in TUBO tumors within the same animal. Addition of anti-CD137 mAb to cetuximab increased NK cell and DC infiltrate in TUBO-EGFR tumors and CD8 T cells infiltrate in both TUBO-EGFR and TUBO tumors. Mice treated with combination therapy had more tumor specific circulating CD8 T cells (as measured by IFN-γ production after in vitro restimulation with TUBO and TUBO-EGFR cells) than mice treated with cetuximab alone. These observations strongly support the potential of anti-CD137 mAb to enhance the adaptive CD8+ cytotoxic T cell response and epitope spreading beyond EGFR when combined with cetuximab.
The ability of anti-CD137 to enhance the “vaccinal effect” of cetuximab may act at different levels (). First, CD137 is expressed on NK cells upon activation by anti-tumor Abs through their FcRs. Thus, anti-CD137 can increase ADCC resulting in more tumor lysis and the release of tumor antigens (Ag). Second, DC can express CD137. DC that have been recruited at the tumor site may thus be stimulated by anti-CD137 to present tumor Ags and generate/expand anti-tumor T cells. DC stimulation may also be enhanced through the cross-talk between NK cells and DC. In vitro, we have shown that, in the presence of activated NK cells, EGFR-expressing cancer cells, cetuximab and immature DC, anti-CD137 agonistic Ab enhanced secretion of IL-12, IFN-γ and TNF-α.Citation8 Third, anti-CD137 may also stimulate anti-tumor T cells and, in turn, enhance the adaptive immune response for cytotoxicity, epitope spreading and memory.
Figure 1. Enhancement of the vaccinal effect of an anti-tumor Ab (directed against EGFR) by an anti-CD137 antibody: presumed mechanisms of action. (A) Anti-EGFR mAb binds to EGFR-positive tumor cells and recruit NK cells and DC through their FcR. NK cells mediated ADCC is enhanced by CD137 stimulation and leads to tumor lysis and release of tumor antigens. (B) Tumor-antigens are presented to T cells by DC. This presentation may be facilitated by CD137 stimulation (expressed on DC) and cross-talk with NK. (C) Anti-tumor T cells are generated and expanded following Ag presentation. They can be further stimulated by anti-CD137 mAb. (D) Anti-tumor T cells generated in the microenvironment of EGFR-positive tumor cells can migrate to distant tumor sites, eradicate EGFR-negative tumor cells (through epitope spreading) and retain memory.
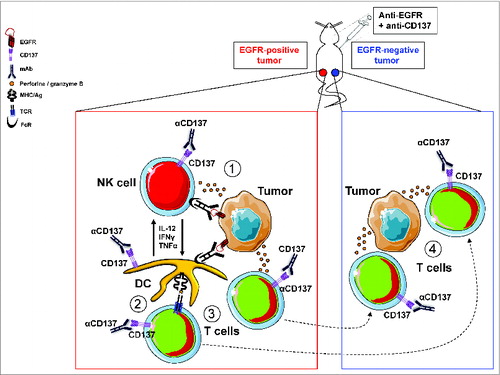
The recent success of immunomodulating mAbs such as those directed against CTLA4 and PD1/PDL1 opens an exciting new field in cancer immunotherapy. Compared to other immunomodulating antibodies, anti-CD137 mAb may be unique because it can target both innate and adaptive immune cells. The combination of anti-CD137 with an anti-tumor mAb may indeed enhance ADCC, Ag presentation and T cell response. Therefore, anti-CD137 mAb can enhance the efficacy of tumor-directed mAbs 1) by stimulating the innate, short lasting response and 2) by inducing an active, polyclonal, and long lasting immune response against tumor thereby reducing the risk of tumor-escape and relapse. These results further support the therapeutic potential of combining monoclonal Abs to target simultaneously the tumor and its immune environment. These preclinical results will have to be confirmed in patients. Several clinical trials are ongoing to test the combination of anti-CD137 mAb and anti-tumor mAbs (NCT01775631, NCT01775631 and NCT01307267). A specific monitoring (including analysis of tumor infiltrate, DC maturation in tumor sites, EGFR tetramer-positive CD8+ T cells, and T cell repertoire) will be performed in the trial testing the combination cetuximab and anti-CD137 mAb (NCT01775631) to measure the induction/enhancement of the adaptive immune response.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317-27; PMID: 20414205; http://dx.doi.org/10.1038/nri2744
- Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood 2004; 104:2635-42; PMID: 15226177; http://dx.doi.org/10.1182/blood-2004-03-1110
- Abès R, Gélizé E, Fridman W H, Teillaud J-L. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood 2010; 116:926-34; PMID: 20439625; http://dx.doi.org/10.1182/blood-2009-10-248609
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18:160-70; PMID: 20708157; http://dx.doi.org/10.1016/j.ccr.2010.06.014
- Selenko N, Majdic O, Jäger U, Sillaber C, Stöckl J, Knapp W. Cross-priming of cytotoxic T cells promoted by apoptosis-inducing tumor cell reactive antibodies? J Clin Immunol 2002; 22:124-30; PMID: 12078853; http://dx.doi.org/10.1023/A:1015463811683
- Srivastava RM, Lee SC, Filho PAA, Lord CA, Jie H-B, Davidson HC, López-Albaitero A, Gibson SP, Gooding WE, Ferrone S, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res Off J A. Assoc Cancer Res 2013; 19:1858-72; http://dx.doi.org/10.1158/1078-0432.CCR-12-2426
- Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, Fisher RI, Kelleher Jr RJ, Bankert RB, Bernstein SH. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a ‘vaccinal effect’ of rituximab. Blood 2009; 113:3809–12; PMID: 19196657; http://dx.doi.org/10.1182/blood-2008-10-185280
- Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 2014. http://dx.doi.org/10.1172/JCI73014
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011; 117:2423-32; PMID: 21193697; http://dx.doi.org/10.1182/blood-2010-08-301945
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng W-K, Clarke MF, Carlson RW, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012; 122:1066-ndash;75; PMID: 22326955; http://dx.doi.org/10.1172/JCI61226
