Abstract
We recently identified tumor-associated macrophages from pancreatic ductal adenocarcinoma sharing pro- and anti-inflammatory characteristics. Already in residence in the setting of chronic pancreatitis, local macrophages confer malignancy-associated features to premalignant pancreatic ductal epithelial cells by both promoting and inhibiting inflammation, either of which can foster malignant conversion. Our findings support the concept that contrasting modes of inflammation can promote tumorigenesis.
Besides their fundamental role in immunity, inflammation and tissue remodelling, macrophages are integral to tumorigenesis, including tumor-associated inflammation.Citation1 Originating from monocytes that extravasate from the circulation into the tissues, macrophages differentiate in response to environmental factors. Analogous to the dichotomous differentiation of T helper cells giving rise to T helper type 1 (Th1) and T helper type 2 (Th2) T cells with distinct cytokine expression patterns and immunological functions, 2 fundamental macrophage phenotypes have been delineated. M1- macrophages, also termed classically activated or pro-inflammatory macrophages, develop in response to bacterial products or interferon-g (IFN-γ). M1-macrophages are characterized by the release of pro-inflammatory cytokines and chemokines, such as the interleukins IL-1β and IL-6, as well as tumor necrosis factor a (TNF-α) and are further distinguishable by a high capacity for antigen presentation imparted by elevated HLA-DR expression. In contrast, M2-macrophages, also termed alternatively activated or anti-inflammatory macrophages, develop in response to IL-4, IL-13 or glucocorticoids and are characterized by secretion of anti-inflammatory mediators, such as transforming growth factor b (TGF-β1) and IL-10, as well as by their ability to promote extracellular matrix remodelling and angiogenesis.Citation2 Accordingly, the latter phenotype has been primarily associated with tumor development and progression so that tumor-associated macrophages (TAMs) have been declared as M2-macrophages. Supporting this view, a high abundance of M2-related markers (e.g. CD163, CD204) in tumor tissues have been shown to negatively correlate with survival of tumor patients, including pancreatic ductal adenocarcinoma (PDAC) patients.Citation3 However, most of these studies have relied either on the characterization of TAMs by immunohistochemistry of single M2-markers, or on investigations of their functional impact on tumor cells predominantly in mouse models. Our recent work provides novel insights into the phenotype and impact of TAMs in human PDAC.Citation4 Using TAMs freshly isolated from patient-derived PDAC tissues, comprehensive analyses were conducted regarding their phenotype and functional impact on premalignant (H6c7) and malignant (Colo357) pancreatic ductal epithelial cells.Citation4 Flow cytometry and real-time PCR based analysis revealed that human PDAC-associated TAMs concomitantly exhibit characteristics of pro-inflammatory M1-macrophages, exemplified by elevated expression of HLA-DR, IL-1β and TNF-α, as well as of those of anti-inflammatory M2-macrophages, exemplified by elevated expression of CD163 and IL-10. Moreover, HLA-DR and CD163 double positive cells were detected by immunohistochemical stainings in pancreatic tissues of PDAC and chronic pancreatitis (CP) patientsCitation5 confirming that macrophages with a mixed phenotype sharing pro- and anti-inflammatory properties are abundant in PDAC and are already present in CP settings. While equally distributed in CP tissues, macrophages were predominantly localized close to the cancerous epithelium in PDAC tissue. This local enrichment of TAMs correlated with higher tumor grading and an elevated expression of the mesenchymal protein vimentin in cancer cells, indicating epithelial-mesenchymal transition (EMT). Although being localized in close proximity to the cancerous epithelium, macrophages were hardly detected in direct cell-cell contact with the tumor cells, supporting the view that macrophages contribute to EMT in pancreatic epithelial cells mainly in a paracrine manner.Citation5 In line with these findings, H6c7 and Colo357 cells acquired an elongated cell shape along with an increased expression of vimentin and a reduced expression of epithelial E-cadherin when indirectly cocultured with TAMs from PDAC tissues.Citation4 To elucidate whether pro- or anti-inflammatory properties of macrophages account for these effects, H6c7 and Colo357 cells were cocultured in the presence of in vitro polarized M1- and M2-macrophages. Similar to TAMs, both macrophage subsets induced EMT-associated alterations in H6c7 and Colo357 cells and even greater invasiveness of Colo357 cells. Elevated levels of TNF-α and TGF-β1 in supernatants of co-cultured H6c7 and Colo357 cells point to a substantial role of these factors in the malignant alterations of both cell lines. Interestingly, similar effects could be observed with colonic epithelial cells supporting the role of macrophages along with their pro- as well as anti-inflammatory abilities in EMT and their general impact in tumorigenesis.Citation6
Altogether, our findings corroborate the well-established role of macrophages as “obligate partners for tumor cell migration, invasion and metastasis”.Citation7 Importantly, this study substantially adds to the current view on the heterogeneity of macrophages in tumor tissues and challenges the dichotomous concept of M1- and M2-macrophages -at least in regards to TAMs. This interpretation is in line with other findings showing that the phenotype and function of TAMs are essentially determined by their spatial localization within in the tumor.Citation8 Moreover, our data clearly demonstrate that macrophages can exert tumor-promoting activities, such as inducing EMT in the pancreatic ductal epithelium, by exerting pro-inflammatory properties of similar magnitude, or even stronger than, their pronounced anti-inflammatory phenotype, substantiating the view that distinct modes of inflammation contribute to tumor initiation and progression ().
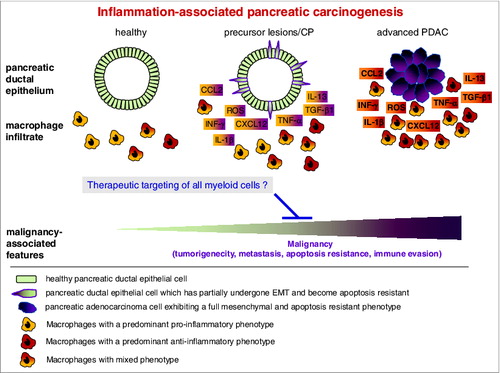
Figure 1. Macrophages with pro- and anti-inflammatory properties contribute to pancreatic tumorigenesis. In the course of inflammation-associated initiation and progression of pancreatic ductal adenocarcinoma, macrophages exhibiting distinct characteristics accumulate in pancreatic tissues, some of which are localized in close proximity to the cancerous epithelium. Upon this mutual interaction, a plethora of pro- and anti-inflammatory cytokines (e.g., IFN-γ, IL-1β, IL-13, TNF-α, TGF-β1), chemokines (CCL2, CXCL12) and reactive oxygen species (ROS) are released by carcinoma cells as well as by the heterogenic population of macrophages. In chronic pancreatitis or pancreatic ductal adenocarcinoma (PDAC), these factors account for the acquisition of malignancy-associated alterations in the conversion of pancreatic epithelial to carcinoma cells, on the one hand, while reciprocally contributing to the shaping of the phenotype and function of the macrophages on the other hand. Accordingly, therapeutic strategies targeting all myeloid cells might represent effective weapons to comprehensively impair tumorigenicity, metastasis, apoptosis resistance and immune evasion in PDAC patients.
Given the high abundance of macrophages in CP tissues and their impact already on premalignant pancreatic ductal epithelial cells, EMT and subsequent pancreatic cell dissemination might occur quite early in pancreatic tumorigenesis, as suggested by recent observations in an endogenous PDAC mouse model.Citation9
Altogether these findings raise the question of the optimal strategy for targeting macrophages in anticancer therapy. Concepts aiming to re-polarize TAMs into a more pro-inflammatory phenotype may be less effective, or even deleterious, when considering the fact that the favorable immunomodulatory effects are compensated for by the direct tumorigenic activity of these macrophages on tumor cells. Such strategies should be regarded therefore with some caution and as being applicable only for certain tumor entities or in combination with other therapeutics. More promising and effective seem to be strategies that selectively target all macrophage subsets, as recently reported for the commercially available chemotherapeutic agent trabectedin.Citation10 This drug is already in use for the treatment of a small number of oncological indications, mostly as second line treatment, but in light of our progress in understanding the how broadly TAMs can promote tumorigenesis, such macrophage targeting drugs require further clinical exploration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008; 454:428-35; PMID: 18650913; http://dx.doi.org/10.1038/nature07201
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18:349-55; PMID: 18467122; http://dx.doi.org/10.1016/j.semcancer.2008.03.004
- Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol 2013; 4:210; PMID: 23950747; http://dx.doi.org/10.3389/fphys.2013.00210
- Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren U, Vogel I, Krüger U, Becker T, Ebsen M, et al. Tumor associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014; 135:843-61; PMID: 24458546; http://dx.doi.org/10.1002/ijc.28736
- Helm O, Mennrich R, Petrick D, Goebel L, Freitag-Wolf S, Röder C, Kalthoff H, Röcken C, Sipos B, Kabelitz D, Schäfer H, et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One. 2014; 9:e94357; PMID: 24797069; http://dx.doi.org/10.1371/journal.pone.0094357
- Schäfer H, Struck B, Feldmann E-M, Bergmann F, Grage-Griebenow E, Geismann C, Ehlers S, Altevogt P, Sebens S. TGF-b1 dependent L1CAM expression plays an essential role in macrophage induced apoptosis resistance and cell migration of human intestinal epithelial cells. Oncogene 2013; 32:180-9; PMID: 22349829; http://dx.doi.org/10.1038/onc.2012.44
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124:263-6; PMID: 16439202; http://dx.doi.org/10.1016/j.cell.2006.01.00
- Rivera LB, Bergers G. Location, location, location: macrophage positioning within tumors determines pro- or antitumor activity. Cancer Cell 2013; 24:687-9; PMID: 24332035; http://dx.doi.org/10.1016/j.ccr.2013.11.014
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148:349-61; PMID: 22265420; http://dx.doi.org/10.1016/j.cell.2011.11.025
- Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013; 23:249-62; PMID: 23410977; http://dx.doi.org/10.1016/j.ccr.2013.01.008
