Abstract
Preclinical models have been developed and applied to predict the clinical efficacy of immune checkpoint antibodies. Now these models can be used to dissect the mechanisms by which such immunotherapeutic antibodies work and to build the rationale for combining immune checkpoint-targeting antibodies with potential synergistic activity in cancer patients.
Authors' View
The antitumor efficacy of anti-CTLA-4 monoclonal antibody was originally explained by its blockade of a negative homeostatic signal delivered through cytotoxic T lymphocyte associated protein 4 (CTLA-4) on the T effector (Teff) cell surface. This concept, primarily based on the unconstrained T-cell proliferation rampant in the CTLA4 knockout (CTLA4-/-) mouse, has recently been challenged by results implicating a role for negative regulatory cells present in the tumor microenvironment targeted for elimination by anti-CTLA4 antibodies.
Intratumoral Treg Cells are Depleted During Therapy with Antibodies Against CTLA-4, OX40 and GITR
Our group, and those of Selby et al., Bulliard et al., and Simpson et al., have all demonstrated in various tumor models that the effectiveness of anti-CTLA-4 therapy is dependent upon its ability to deplete regulatory T cells (Tregs) residing within the tumors.Citation1-4 Using different isotypes of the same anti-CTLA-4 monoclonal antibody (mAb), Selby et al. showed that CTLA-4 blockade, alone, was insufficient to obtain efficient antitumor responses.Citation4 In 2 different adenocarcinoma models (CT26 in Balb/c mice and MC38 in C57Bl6 mice), they showed that although monotherapy with the IgG2a anti-CTLA-4 isotype cured 90–100% of tumor-bearing mice, all mice died of tumor progression when anti-CTLA-4 antibody of either IgG1 or IgG2b isotypes were employed. Antibodies of the IgG2a isotype are effective in mediating antibody dependent cellular cytotoxicity (ADCC) and antibody dependent cellular phagocytosis (ADCP) in mice, whereas IgG1 and IgG2b are not. Therefore, this IgG2a dependency suggested that ADCC or ADCP, rather than signal blockade was involved in this antitumor effect. This hypothesis was bolstered by experiments performed by Simpson et al. and Bulliard et al. showing that the anti-CTLA-4 efficacy was lost in Fc common γ chain null mice that have impaired ADCC function. Similarly, the therapeutic efficacy of antibodies against tumor necrosis factor receptor superfamily members (Tnfrsf4, better known as OX40) and (Tnfrsf18, better known as GITR) also were shown to depend on ADCC activity.Citation1,3,5 Both Simpson et al. and Bulliard et al. subsequently demonstrated that this antibody-mediated Treg depletion was dependent specifically on FcγRIV, an activating Fc receptor selectively expressed on intratumoral CD11b+ myeloid cells.Citation2,3 In contrast, the antitumor efficacy of these same mAbs was not found to involve FcγRIIb. This conclusion appears to be contradictory to recent results showing that the antitumor efficacy of anti-CD40 and anti-DR5 immune checkpoint antibodies rely on FcγRIIb.Citation6 However, the apparent discrepancy in route of action between these groups of mAbs could be explained by the differences in their isotypes affinity (IgG1 versus IgG2a) for these 2 different Fc receptors.
Intratumoral Tumor-antigen Specific Tregs Co-express High Levels of Immune Checkpoint Proteins
A phenotypic analysis of T cells in tumor-bearing mice revealed that CTLA-4, OX40 and GITR are highly expressed on the surface of intratumoral Tregs, but not on Tregs located in other sites of the body, notably including T cells residing in the tumor draining lymph nodes.Citation1-3 The majority of intratumoral Tregs coexpressed both CTLA-4 and OX40.Citation1 Interestingly, in transgenic mice expressing OVA peptide T cell receptor (TCR) and bearing 2 lymphoma tumors, one wild type and one expressing the OVA peptide, we could show that OX40 and CTLA-4 were upregulated only within the OVA-expressing tumor and only on the surface of the OVA antigen-specific Tregs (i.e., not on the OVA-specific Teff cells). When these OVA-expressing tumors were treated with antibodies against OX40 & CTLA-4 these OVA-specific Tregs were preferentially depleted. Simpson et al. also demonstrated a similar phenomenon in the B16 melanoma tumor model, where they found that only tumor-antigen specific Tregs upregulated CTLA-4 and were depleted upon anti-CTLA-4 therapy. Interestingly, the OVA-specific system allowed us to show that anti-OX40 and anti-CTLA-4 therapy selectively depleted the OVA-specific Tregs while sparing the OVA-specific Teff cells within OVA-expressing tumors.Citation1
Overcoming Resistance to Immune Checkpoint Antibody Monotherapy by Synergistic Combinations with Immunostimulatory Products
Our group previously showed that lymphoma tumors were resistant to immune checkpoint monotherapy but could be treated by local injections of CpG oligodeoxynucleotides, a ligand for the Toll-like receptor 9 (TLR9).Citation7 We found that antibodies against a variety of checkpoint targets could augment the therapeutic effect of intratumoral CpG, including anti-CTLA-4, anti-OX40 or anti-GITR.Citation7 Surprisingly, the combination of intratumoral CpG together with certain combinations of these antibodies was very effective and could eradicate tumors at both the injected site, and remarkably, at distant non-injected sites. Similarly, Simpson et al. also showed that anti-CTLA-4 single agent therapy was inefficient in the C57Bl6 B16 melanoma model. However, this resistance could be overcome when anti-CTLA-4 was used in combination with a tumor-cell based vaccine secreting the immunostimulatory cytokine granulocyte macrophage colony-stimulating factor (GM-CSF).Citation2 More recently, Zamarin et al. also showed that the combination of systemic anti-CTLA-4 with an intratumoral oncolytic virus could overcome resistance to anti-CTLA-4 monotherapy.Citation8
Targeting Immune Tolerance Locally to Trigger Systemic Antitumor Immunity
Because OX40 and CTLA-4 are predominantly expressed on intratumoral, tumor-specific Tregs, we tested whether the antibodies against CTLA-4 and OX40 could be injected into one tumor site along with the CpG. We found that, this combination of in situ immunomodulators triggered a CD4+ and CD8+ T-cell mediated anti-tumor immune response that was able to eradicate distant, non-injected, tumors including those implanted in the brain. Surprisingly, these mice treated by combined intra-tumoral therapy were even better protected against late tumor relapses than were the mice that received the anti-OX40 and anti-CTLA-4 antibodies by a systemic route. These long-term surviving mice developed a mAb therapy-induced CD8+ mediated antitumor T-cell immunity protecting them against tumor re-challenge, even in the brain.
Of note, the intratumoral injection strategy provided the same therapeutic efficacy with antibody doses as low as 1% of the required systemic doses. The ability of peritumoral low dose anti-CTLA-4 antibody to trigger systemic antitumor immunity has also been independently demonstrated recently by Fransen et al. in another tumor model.Citation9 Importantly, this low dose strategy prevents the systemic exposure to immune checkpoint mAbs and their off-target auto-immune toxicity.Citation1,9,10
The intratumoral dosing strategy allowed us to demonstrate that the Treg depletion occurs only in the injected sites but not within the distant uninjected tumor sites. This observation suggests that the local intratumoral immunosuppressive phenotype predominantly blocks the initiation of an immune response, as opposed to the therapeutic potency of Teff cells once they arrive at sites of distant disease, where the tumor microenvironment has not yet been significantly modified.
Taken together, and as shown in , these pre-clinical results imply that the mechanism of action of antibodies against CTLA-4 and other immunomodulatory antibodies is through the depletion of intratumoral tumor-specific Tregs. Further, they provide rationale for combinatorial early phase clinical trials to improve the response rate and survival of patients undergoing immune checkpoint blockade therapy.
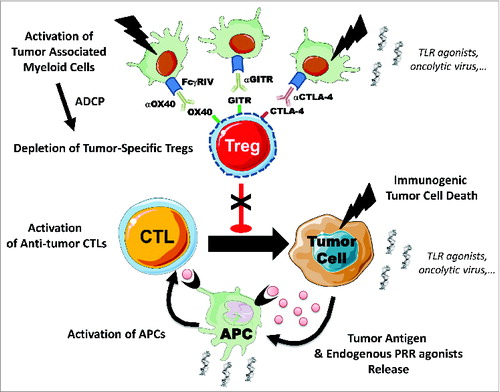
Figure 1. Immune checkpoint antibodies deplete tumor-specific Tregs in situ. Intratumoral regulatory T cells (Tregs) with tumor-antigen specificity overexpress cytotoxic T lymphocyte associated protein 4 (CTLA-4), and tumor necrosis factor receptor superfamily members OX40 (Tnfsrf4) and GITR (Tnfsrf18) on their surface. Anti-CTLA-4, anti-OX40 and anti-GITR immune checkpoint antibody therapies could deplete intratumoral Tregs by antibody dependent cellular phagocytosis (ADCP) via activating FcgRIV receptors expressed on tumor-associated myeloid cells. This depletion might be insufficient to unleash the anticancer immune response in some tumor contexts. Combinations of immune checkpoint monoclonal antibodies (mAbs) with other immunostimulatory products, such as pattern recognition agonists (PRR), or cytotoxic agents activating myeloid cells and/or immunogenic cell death could potentially dramatically enhance the antitumor immune response. APC, antigen presenting cell; CTL, cytotoxic T lymphocyte; TLR, Toll-like receptor.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell R, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013; Jun 3; 123:2447-63; PMID:23728179; http://dx.doi.org/10.1172/JCI64859
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695-710; PMID:23897981; http://dx.doi.org/10.1084/jem.20130579
- Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. Rockefeller University Press; 2013; 210:1685-93; PMID:23897982; http://dx.doi.org/10.1084/jem.20130573
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013; 1:32-42; PMID:24777248; http://dx.doi.org/10.1158/2326-6066.CIR-13-0013
- Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol 2014; 92:475-80; PMID:24732076; http://dx.doi.org/10.1038/icb.2014.26
- Li F, Ravetch J V. Antitumor activities of agonistic anti-TNFR antibodies require differential FcγRIIB coengagement in vivo. Proc Natl Acad Sci U S A 2013; 110:19501-6; PMID:24218606; http://dx.doi.org/10.1073/pnas.1319502110
- Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood 2009; 113:3546-52; PMID:18941113; http://dx.doi.org/10.1182/blood-2008-07-170274
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 2014; 6:226ra32-226ra32; PMID:24598590; http://dx.doi.org/10.1126/scitranslmed.3008095
- Fransen MF, van der Sluis TC, Ossendorp F, Arens R, Melief CJM. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res 2013;19:5381-9; PMID:23788581; http://dx.doi.org/10.1158/1078-0432.CCR-12-0781
- Simmons AD, Moskalenko M, Creson J, Fang J, Yi S, VanRoey MJ, Allison JP, Jooss K. Local secretion of anti-CTLA-4 enhances the therapeutic efficacy of a cancer immunotherapy with reduced evidence of systemic autoimmunity. Cancer Immunol Immunother 2008;57:1263-70; PMID:18236040; http://dx.doi.org/10.1007/s00262-008-0451-3
