Abstract
Single agent immunotherapy is effective against several cancers, but has failed against poorly immunogenic cancers, including pancreatic cancer. Evaluation of pancreatic tumors following treatment with an experimental vaccine (Lutz et al. Cancer Immunology Research 2014) suggests that vaccination primes the tumor microenvironment (TME) for checkpoint-inhibitor immunotherapy, and supports a new platform for evaluating checkpoint-inhibitors in poorly immunogenic cancers.
Pancreatic ductal adenocarcinoma (PDAC) is highly resistant to chemotherapy and radiation therapy. While surgical resection provides the best opportunity for a cure, only around 20% of patients are candidates for surgery, and as many as 80% of patients recur following surgical resection and adjuvant therapy. Median survival for patients with unresectable metastatic PDAC remains <1 y, and the overall 5 y survival rate for PDAC is only 6%.Citation1
Cancer immunotherapy is considered to be one of the biggest breakthroughs for cancer treatment in the last decade. Ipilimumab, a monoclonal antibody that blocks the immune checkpoint cytotoxic T lymphocyte antigen-4 (CTLA-4) was approved by the United States FDA for the treatment of advanced melanoma.Citation2 More recently, other checkpoint inhibitors including Programmed-Death-1 (PD-1) and Programmed-Death-1 Ligand-1 (PD-L1) blocking antibodies were shown to induce objective responses in approximately 20–30% of patients with several cancers, including melanoma, renal cell carcinoma, and non-small cell lung cancer (NSCLC).Citation3-5 Despite the success of blocking CTLA-4 and PD-1 as single therapy in several cancers, treatment of patients with PDAC with these single agents has been ineffective.Citation6
One difference between tumors that have responded to checkpoint-inhibitors and PDAC is the immune status of the TME. Cancers that have responded to checkpoint-inhibitors tend to be naturally infiltrated with effector lymphocytesCitation7 and are generally considered to be `immunogenic’ neoplasms. PDAC, on the other hand, is similar to many other solid malignancies. Instead of being infiltrated with high numbers of effector lymphocytes, PDAC is characterized by a highly immunosuppressive TME that is infiltrated with multiple immunosuppressive regulatory cells.Citation8 As a result, cancers like PDAC are generally considered to be `non-immunogenic’ neoplasms, which has slowed the development and application of immune-based therapies for these diseases.
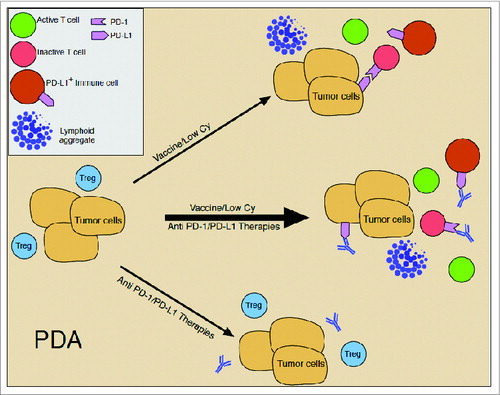
Figure 1. Model explaining the inefficacy of single agent immunotherapy for pancreatic cancer. At baseline, pancreatic tumors are predominantly infiltrated with immunosuppressive regulatory cells, such as T regulatory cells (Treg), and few effector T cells (T cell); express low levels of PD-L1 on tumor cells; and are infiltrated with few to no PD-L1-expressing innate immune cells (PD-L1+ immune cell). In this non-immunized and inactive state, treatment with immune checkpoint inhibitors alone, such as anti-PD-1/PD-L1, is hampered by the lack of effector T cells to act on. Treatment with the GVAX vaccine (Vaccine) combined with low dose cyclophosphamide (Cy) converts the pancreatic tumor microenvironment from a relatively inactive to an active state by inducing the infiltration of effector T cells and the formation of intratumoral tertiary lymphoid aggregates (Lymphoid aggregate). However, cytokines produced by the immune cells that are induced to traffick the tumor, such as IFNγ, induce the upregulation of immunosuppressive mechanisms, such as the upregulation of PD-1 and PD-L1 expression. These countering immunosuppressive mechanisms limit the activity and efficacy of vaccination, but also prime the pancreatic tumor microenvironment for immune modulators, such as checkpoint-inhibitors. Thus, optimal activity and antitumor efficacy of either of these single approaches is dependent on the other.
Our group has developed a vaccine (GVAX) for the treatment of PDAC consisting of two allogeneic PDAC cell lines engineered to secrete GM-CSF.Citation9,10 GVAX is designed to induce immune responses against a broad range of PDAC-associated antigens, including the commonly expressed PDAC antigen mesothelin. Studies evaluating GVAX in patients with both resected and metastatic PDAC have shown that GVAX induces enhanced mesothelin-specific T cell responses in a subset of patients that are associated with longer survival.Citation11,12 Prior work has also shown that combining GVAX with low-dose cyclophosphamide (Cy) to deplete CD4+ T regulatory cells (Tregs) results in more robust mesothelin-specific T cell responses and longer survival in patients with metastatic PDAC compared to GVAX alone.Citation11 Although our prior work demonstrates that GVAX treatment induces peripheral T cell responses that can be enhanced with low-dose Cy, the studies were not designed to directly evaluate the effects of GVAX treatment on the PDAC TME. Therefore, we designed a neo-adjuvant and adjuvant clinical trial comparing GVAX given as single agent, or in combination with low dose Cy.Citation13 The first treatment was given 2 weeks prior to surgery providing the first opportunity to study how the PDAC TME is altered by GVAX-based immunotherapy.
Immunohistochemical analysis (IHC) of resected tumor tissue revealed the formation of intratumoral tertiary lymphoid aggregates in 33 (84.6%) of 39 vaccinated patients that are not observed in tumors from GVAX-naive patients. The aggregates were composed of naïve and activated T cells, B cells and innate antigen-presenting cells (APCs); and resembled ectopic lymph node-like structures observed in immunotherapy naïve patients with melanoma, colon cancer and NSCLC.Citation14-17 Lymphoid aggregates formed regardless of whether GVAX was given with or without Cy. However, lower numbers of FoxP3+ Tregs were observed in tumors from patients treated with the combination of GVAX+Cy indicating that low-dose Cy reduces Treg levels within the TME. In contrast to primary and secondary lymphoid structures, tertiary lymphoid structures develop in response to antigen exposure.Citation18 Thus, their formation demonstrates that GVAX induces an adaptive immune response within the PDAC TME.
Treatment with GVAX induced interferon gamma (IFNγ)-production in T effector cells infiltrating PDACs, but also induced the upregulation of immunosuppressive regulatory mechanisms, including upregulation of the PD-1/PD-L1 pathway(). In unvaccinated patients, only a small percentage of PDAC tumor cells expressed low levels of membranous PD-L1. By contrast, moderate membranous expression of PD-L1 by tumor cells was observed in patients treated with GVAX. Lymphoid aggregates were also infiltrated with innate immune cells expressing high levels of PD-L1. Although PD-L1 expression may be regulated by oncogenic pathways, PD-L1 is also induced by cytokines produced by infiltrating immune cells, such as IFNγ. Citation19 In immunotherapy-naive patients with melanoma, NSCLC and renal cell carcinoma, PD-L1 expression has been observed in approximately 53–89% of tumors and by infiltrating immune cells in approximately 50–100% of tumors.Citation7 The expression of PD-L1 in tumors is associated with more abundant immune cell infiltration and the presence of lymphoid aggregates. The naturally high prevalence of PD-L1 in these tumor types may explain their relatively high response rates to anti-PD-1 or anti-PD-L1 Citation3-5; whereas the low PD-1/PD-L1 levels expressed by PDAC may explain why these agents have been less effective against PDAC. However, by inducing T cell infiltration and PD-L1 expression in the TME, GVAX may prime PDACs for anti-PD-1/PD-L1 therapies.
This study demonstrates that GVAX can convert a `non-immunogenic’ neoplasm into an `immunogenic’ neoplasm by inducing infiltration of T cells and development of tertiary lymphoid structures. However, this conversion coincides with the upregulation of immunosuppressive regulatory mechanisms. These data may explain why GVAX and checkpoint-inhibitors given alone have failed against PDAC, but importantly, also suggest that vaccine-primed PDAC patients may be better candidates for checkpoint immunotherapy than vaccine-naïve patients (). In support of this notion, we recently showed that the combination of GVAX with ipilimumab induces objective responses in patients with metastatic PDAC that are not observed with either single therapy alone.Citation20 These data support a new approach for evaluating checkpoint inhibitors in ``non-immunogenic" cancers, like PDAC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: Cancer J Clini 2014; 64(1):9-29; http://dx.doi.org/10.1017/S0009840X13002084
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med 2012; 366(26):2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 2012; 366(26):2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New Engl J Med 2013; 369(2):134-44; PMID:23724846; http://dx.doi.org/10.1056/NEJMoa1305133
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33(8):828-33; PMID:20842054; http://dx.doi.org/10.1097/CJI.0b013e3181eec14c
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20(19):5064-74; PMID:24714771
- Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterol 2013; 144(6):1230-40; http://dx.doi.org/10.1053/j.gastro.2012.12.042
- Jaffee EM, Schutte M, Gossett J, Morsberger LA, Adler AJ, Thomas M, Greten TF, Hruban RH, Yeo CJ, Griffin CA. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am 1998; 4(3):194-203.; PMID:9612602
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol: Off J Am Soc Clin Oncol 2001; 19(1):145-56
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res: Off J Am Assoc Cancer Res 2008; 14(5):1455-63; http://dx.doi.org/10.1158/1078-0432.CCR-07-0371
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011; 253(2):328-35; PMID:21217520; http://dx.doi.org/10.1097/SLA.0b013e3181fd271c
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014; 2(7):616-31; PMID:24942756; http://dx.doi.org/10.1158/2326-6066.CIR-14-0027
- Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72(16):3997-4007; PMID:22850419; http://dx.doi.org/10.1158/0008-5472.CAN-12-1377
- Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mule JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol 2011; 179(1):37-45; PMID:21703392; http://dx.doi.org/10.1016/j.ajpath.2011.03.007
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol: Off J Am Soc Clin Oncol 2008; 26(27):4410-17; http://dx.doi.org/10.1200/JCO.2007.15.0284
- Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS, Mule JJ. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep 2012; 2:765; PMID:23097687; http://dx.doi.org/10.1038/srep00765
- Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunol 2013; 2(12):e26836; http://dx.doi.org/10.4161/onci.26836
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012, 4(127):127ra137; http://dx.doi.org/10.1126/scitranslmed.3003689
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr., Donehower RC, Jaffee EM et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013; 36(7):382-9; PMID:23924790; http://dx.doi.org/10.1097/CJI.0b013e31829fb7a2
