Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality and has increasingly become a disease of elderly patients. Elderly patients are underrepresented in clinical trials that evaluate treatments for NSCLC. It has been suggested that patients >65 years of age have less robust immune responses to infections, immunizations, and tumors compared with younger patients. With increasing focus and number of immunotherapy clinical trials for NSCLC, we investigated the relationship between patient age and the tumor immune microenvironment in NSCLC. Using tissue microarrays from 1,278 patients with surgically resected Stage I NSCLC (≤65 years [33%], 66–79 years [55%], and ≥80 years [12%]), we determined whether quantitative and qualitative immune cell infiltration in the tumor differed between younger and older patients. Furthermore, we investigated the prognostic value of immune cell infiltration with respect to recurrence in octogenarians. We found that there were no statistically significant differences between older and younger patients in tumoral immune infiltration or effector regulatory immune response ratios (FoxP3/CD3, FoxP3/CD4, and FoxP3/CD8 ratios). In octogenarians, presence of low tumoral CD68+ immune cells was an independent predictor of recurrence. In the uniform cohort of surgically selected and resected Stage I NSCLC patients, tumor immune cell infiltration among the older age group resembled other age groups. Our study provides information that supports inclusion of older age patients selected for surgical resection in neoadjuvant or adjuvant immunotherapy clinical trials for lung cancer.
Introduction
Lung cancer, which is the leading cause of cancer-related mortality, has increasingly become a disease that afflicts elderly patients.Citation1 Within this group, octogenarians (≥80 years of age) represent the most rapidly growing group of patients with lung cancer.Citation1,2 With the increase in life expectancy in the United States, the elderly population is projected to double from the current estimate of 35 million to 70 million by the year 2030.Citation2,3 In a published study analyzing Surveillance, Epidemiology, and End Results (SEER) data, of all 316,882 patients with lung cancer, 45,912 patients (14%) were ≥80 years of age, 33% were 70–79 years of age, and 53% were <70 years of age.Citation2 In addition, with the anticipated increase in detection of lung cancer via computed tomography (CT) screenings,Citation4-6 an even greater number of elderly patients may be diagnosed with early-stage lung cancer. Elderly patients with lung cancer are also significantly underrepresented in clinical trials; low enrollment of patients older than 70 years of age was largely responsible for this discrepancy.Citation2,7,8 In fact, the European Organization for Research and Treatment of Cancer (EORTC) has stated that this issue is one of their priorities.Citation9
Aging of the immune system, referred to as immunosenescence, is characterized by a decrease in cell-mediated immune function and a reduction of humoral immune responses.Citation10,11 Clinically, immunosenescence is partially responsible for increased prevalence and severity of infectious diseases as well as low efficacy of vaccinations in elderly patients.Citation10 Age-related decreases in immunosurveillance against cancer has also been suggested as a contributing factor to increased cancer rates, such as lung cancer, in elderly patients.Citation12 Furthermore, preclinical studies in animal models have indicated that antitumor immunotherapeutic interventions are less effective in aged animals.Citation13,14
The immune microenvironment is increasingly being recognized as a strong prognostic factor of solid malignancies, including colorectal,Citation15-17 ovarian,Citation18 and breast cancers.Citation19 In a previously published study,Citation20 we reported the biologic and prognostic significance of the tumor immune microenvironment using a large-scale test and validation cohort of Stage I lung adenocarcinoma (ADC) patients. In this study, among patients with high densities of forkhead box P3 (FoxP3+) regulatory T cells, those with high densities of CD3+ T cells had better clinical outcomes than those with low densities of CD3+ T cells. This finding suggests that relative proportions of protumor and antitumor immune responses, as well as immune cell type and density, are important prognostic markers.
With increasing focus on immunotherapy for non-small cell lung cancer (NSCLC), we investigated the relationship between age (≤65 years of age, 66–79 years of age, and ≥80 years of age) and tumor immune parameters in patients with Stage I NSCLC in an effort to: (1) determine whether immune cell infiltration in the tumor differs between elderly and younger patients, (2) determine whether prognosis, with respect to cancer recurrence, of elderly patients is comparable to younger patients after curative-intent surgical resection; and (3) evaluate prognostic value of different types of immune cell infiltration with respect to recurrence in the octogenarians group.
Results
Clinicopathologic factors
A total of 1,278 patients with pathological Stage I NSCLC had undergone complete resection (R0) between 1995 and 2009 (427 were ≤65 years of age, 700 were 66–79 years of age, and 151 were ≥80 years of age) and had adequate tissue available for analysis were included in the study. Clinicopathologic variables for the study cohort are outlined in . Of all 1,278 patients, 1,038 (81%) had lung ADC and 240 (19%) had squamous cell carcinoma (SCC). P-values in represent comparison between clinical variables and 3 age categories. Patients ≤65 years of age were more likely to have lung ADC (89% [n = 378], P < 0.001) than patients in the 66–79 years (78%) and ≥80 years (77%) age groups. Most patients were female (59% [n = 750]) and had Stage IA disease (69% [n = 879]). Patients ≥80 years of age were less likely to be current smokers and more likely to be never smokers compared with patients in the other age groups. Of all patients, 77% (n = 979) had undergone lobectomy and 23% (n = 299) had undergone limited resection. Limited resection was more common in older age patients (≥80 years, 30%; 66–79 years, 24%; and ≤65 years, 19%; P < 0.001).
Table 1. Association between clinicopathologic factors and age group
Tumor-infiltrating immune cells
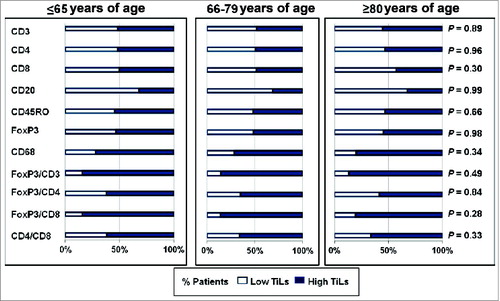
All immune cells were independently assessed for tumor infiltration among the 3 age groups. There were no differences among the 3 age groups in either high or low tumoral immune infiltration of CD3+ (P = 0.9), CD4+ (P = 0.97), CD8+ (P = 0.31), CD20+ (P = 0.99), CD45RO+ (P = 0.67), FoxP3+ (P = 0.98), and CD68+ (P = 0.35) immune cells (). Since FoxP3+ regulatory T cells were protumor immune cells and a subset of the entire T-cell population, we investigated relative proportion of FoxP3+ to CD3+, CD4+, and CD8+ cells among the different age groups. We found no differences in the relative proportions of either FoxP3+ to CD3+ (P = 0.49), FoxP3+ to CD4+ (P = 0.84), or FoxP3+ to CD8+ (P = 0.28) tumor-infiltrating immune cells. Relative proportion of CD4+ to CD8+ was also investigated and found not to be significant (P = 0.34).
Figure 1. Lack of association between immune markers and age groups in non-small cell lung cancer patients. Tissue microarrays were histologically examined to assess potential differences between tumor-infiltrating lymphocytes (TiLs) among the 3 age groups. The P-value was assessed by Cochran-Armitage test (2-sided). *CD68 is a marker for tumor-associated macrophages.
Association between age, histological subtype and recurrence
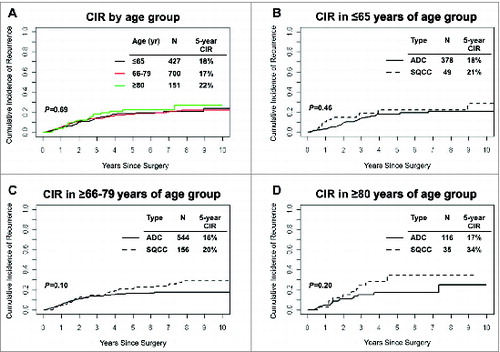
The 5-year cumulative incidence of recurrence (CIR), as a function of age and histological subtypes of NSCLC, is shown in . Risk of recurrence for patients ≥80 years of age (n = 151, 5-year CIR, 22%) was not significantly different than risk of recurrence for patients in the 66–79 (5-year CIR, 18%) and ≤65 years of age groups (5-year CIR, 19%) (). In the ≤65 years of age group, 5-year CIR was comparable in patients with SCC tumors (n = 49, 5-year CIR, 22%) and patients with lung ADC tumors (n = 378, 5-year CIR, 18%) (). In the 66–79 years of age group, 5-year CIR was similar for patients with SCC tumors (n = 156, 5-year CIR, 20%) and patients with lung ADC tumors (n = 544, 5-year CIR, 16%) (P = 0.1) (). Among patients ≥80 years of age, there was no statistically significant difference in risk of recurrence between histological subtypes (5-year CIR = 34% for SCC tumors vs. 5-year CIR = 17% for lung ADC tumors) (P = 0.2) ().
Figure 2. Analysis of disease recurrence and age groups in non-small cell lung cancer patients. (A) Cumulative incidence of recurrence at 5 years, by age group, in Stage I non-small cell lung cancer (n = 1,278). (B-D) Cumulative incidence of recurrence at 5 years by age group and non-small cell lung cancer histologic subtype; (B) ≤65 (C) 66–79 and (D) ≥80 years of age. ADC, adenocarcinoma; SCC, squamous cell carcinoma; CIR, cumulative incidence of recurrence.
Clinicopathological factors, tumor-infiltrating immune cells, and recurrence in octogenarians
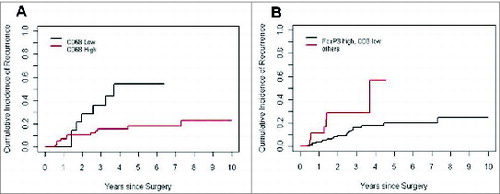
Clinicopathologic factors and their associated CIR for 151 octogenarian patients are shown in . On univariate analysis, limited resection (P = 0.032) and higher disease stage (Stage IB; P = 0.046) were found to be significantly associated with increased CIR. Following this, we assessed each immune marker for its ability to predict recurrence in the octogenarian group (). Among immune cells, low percentages of CD68+ cells (5-year CIR, 54%, p = 0.017) and low relative proportion of FoxP3+ to CD3+ (p = 0.021) were associated with higher risk of recurrence (). None of the other immune cells had a significant prognostic value.
Table 2. Association between clinicopathologic factors and cumulative incidence of recurrence in octogenarians (n = 151)
Table 3. Association between immune markers and cumulative incidence of recurrence in octogenarians (n = 151)
Figure 3. Risk of recurrence in patients with low percentages of CD68+ cells and low relative proportion of FoxP3+ to CD3+ (A) Cumulative incidence of recurrence analyzed according to presence of tumor-infiltrating CD68+ immune cells in non-small cell lung cancer patients ≥80 years of age. (B) Cumulative incidence of recurrence analysis according to FoxP3 high and CD3 low vs. others in patients ≥80 years of age with non-small cell lung cancer.
Multivariate analysis
On multivariate analysis of the octogenarian group, low CD68+ immune cell infiltration, but not low FoxP3+ to CD3+ ratio, remained a significant prognostic factor for recurrence (hazard ratio [HR], 3.21; P = 0.012) (). Neither higher disease stage (hazard ratio [HR], 2.92; P = 0.055) nor type of surgical resection (HR, 1.31; P = 0.56) significantly influenced risk of recurrence.
Table 4. Results of multivariate competing risks regression model
Discussion
WeCitation20-23 and othersCitation15-17,19 have shown the prognostic significance of the tumor immune microenvironment in solid malignancies. To the best of our knowledge, there have been no published studies on the association between tumor-infiltrating immune cells and elderly patients in either NSCLC or other solid malignancies. In the present study of a uniform cohort of patients with Stage I NSCLC, we investigated the 7 most commonly studied markers of immune cell infiltration and found that elderly patients had a tumor immune cell infiltration rate similar to younger patients (). Furthermore, we demonstrated that, in addition to type and density, relative proportion of protumor to antitumor immune cells did not significantly differ between elderly and younger patients (). Published studies have indicated differences in number of tumor-infiltrating immune cells among broad age groups in some solid malignancies.Citation19,24-27 However, these studies were not designed with the explicit purpose of evaluating immune cell infiltration with a specific focus on older patients.
Although 60% of those diagnosed with NSCLC are ≥70 years of age, elderly patients are often undertreated.Citation28,29 Retrospective series suggested that surgery was safe and effective in treating early-stage NSCLC in some patients ≥70 years of age.Citation30-32 Our results suggest that in surgically selected octogenarians outcomes are equivalent to other groups (). For patients with advanced NSCLC, different approaches to immunotherapy have shown promise in early phase clinical trials and have progressed to late-phase development. However, patients ≥70 years of age are underrepresented in lung cancer clinical research trialsCitation33,34 and the role of age-related immunosenescence in the efficacy of immunotherapies remains unclear. With recent results emphasizing number and functionality of tumor-infiltrating immune cells as a critical parameter in predicting the efficacy of immunomodulatory therapies, such as checkpoint inhibitors,Citation35 our findings are of translational importance. Our results support including older patients deemed fit for surgical resection in neoadjuvant or adjuvant immunotherapy trials for lung cancer.
An interesting observation is that there is a higher percentage of never smokers among octogenarians than in other age groups (21% in ≥80 years of age, 12% in 66–79 years of age, and 15% in ≤65 years of age) (). This may indicate that elderly patients investigated in our study were healthy since they were deemed surgically fit for resection and thus may have had good immune status. Our cohort, the largest compilation of Stage I NSCLC of both ADC and SCC investigated to date for immune cell infiltration, also included patients who had undergone limited resection rather than lobectomy. In fact, among elderly patients, a higher percentage of patients (30% in ≥80 years of age, 24% in 66–79 years of age, and 19% in ≤65 years of age) underwent limited resection; this group may have represented patients who were not fit to undergo larger anatomical resections, such as lobectomy. There were no differences in the tumor immune cell infiltration among patients who had undergone limited resection versus lobectomy (data not shown).
Of the investigated immune markers, presence of low tumoral infiltration of CD68+ immune cells in the octogenarian age group correlated with worse clinical outcomes () and was independently associated with risk of recurrence as assessed on multivariate analysis (). Similar to our results, previous work on NSCLC demonstrated that infiltration of CD68-expressing monocytes in the tumor nest was associated with improved clinical outcomes.Citation36,37 However, the fact that our results come from a uniform cohort of Stage I NSCLC patients and that we chose recurrence instead of survival as the endpoint, suggested that our findings were more clinically relevant for Stage I patients. Further, our data were also not confounded by other factors that might have influenced survival.
The present study suffered from limitations imposed by its retrospective design from a single institution. In addition, our results on tumor immune cell infiltration came from surgically resected specimens of a selected population with Stage I NSCLC and may not have been representative of all patients with advanced NSCLC. Although it is unclear whether or not immune cell infiltration in small biopsies reflected those in surgically resected specimens, tumor-infiltrating lymphocytes from small biopsy specimens have been shown to predict therapy responses in advanced NSCLC.Citation38 Another limitation of this study was that these findings were not confirmed in a validation cohort. More importantly, although immune cell infiltrates were quantitatively similar, we did not evaluate functionality of infiltrating immune cells, as this can only be evaluated prospectively from fresh tissue. Considering that immunohistochemical analysis cannot confirm functional differences of each immune cell marker between younger and older patients, further experiments using cell lines and animal model are warranted. Nevertheless, our findings from a large cohort of early-stage NSCLC patients with multiple immune cell characterizations may help clinicians make better therapeutic decisions and stratify patients for immunotherapy.
In conclusion, our study demonstrated that, based on a surgically selected cohort of patients with Stage I NSCLC, the tumor immune microenvironment in elderly patients is similar to younger patients. For a population that is anticipated to increase with the advent of lung cancer screening via CT scans, our results shed light on the complex tumor immune microenvironment in elderly patients. More importantly, these findings provide a foundation for future investigations into immunomodulatory therapies for the older age group.
Material and Methods
Patients
The current study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK). We retrospectively reviewed all patients with pathologic Stage I, therapy-naïve NSCLC who underwent surgical resection at our institution between 1995 and 2009. Tumors other than ADC and SCC were not included in the study. Tumor slides and blocks were selected for tissue microarray (TMA) construction. Clinical data were collected from the prospectively maintained Thoracic Surgery Service Lung Cancer Database. Disease stage was assigned on the basis of the seventh edition of the American Joint Committee on Cancer TNM Staging Manual.Citation39 Smoking history was divided into never-smokers (<100 cigarettes over lifetime), former smokers (quit >1 month before resection), and current smokers.Citation40
Histologic evaluation and tissue microarray
All available hematoxylin and eosin stained slides were reviewed by a pathologist (K. K.) who was blind to patient clinical outcomes using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece. It is important to note that we decided to use only tumoral cores of SCCs for the TMAs since it was difficult to separate intermixed tumor and stroma in SCCs.
Formalin-fixed, paraffin-embedded tumor specimens were used for TMA construction, as previously described.Citation20 In brief, the slide with the most severe inflammatory reaction was chosen for each tumor. Then, 2 to 6 representative TMA cores that were obtained from each tumor nest with the most abundant inflammatory reaction (0.6 mm) were made for further immunohistochemical analysis.
Immunohistochemical analysis and scoring of immune markers
Briefly, 4-μm sections from the TMA blocks were deparaffinized in xylene and dehydrated in graded alcohols. The standard avidin–biotin complex peroxidase technique was used for the immunohistochemical stain of immune markers. Sections were stained using a Ventana Discovery XT Automated Immunohistochemical Stainer (Ventana, Tucson, AZ, USA), according to the manufacturer's guidelines. Diaminobenzidine was used as the chromogen and hematoxylin as the nuclear counterstain. Positive control tissues were stained, in parallel, with the study cases. Under high-power field (magnification, 200x), each core was semi-quantitatively scored for degree of immune cell infiltration in the tumor nest, as previously described.Citation20 Scores for each core were averaged to give 1 score per patient, as previously reported.Citation20,41
Statistical analysis
Patients were classified into 3 categories based on their age at time of surgical resection for lung cancer: ≤65 years of age, 66–79 years of age, and ≥80 years of age. Associations between clinical and immune variables and these age categories were analyzed using the Cochran-Armitage trend test.
To investigate risk of recurrence, patients were followed from date of surgery until recurrence, death from any cause or last follow-up, whichever came first. Since traditional Kaplan-Meier probability estimates can be biased when a large number of patients die without recurrence and are subsequently censored, we used competing risk methods for this analysis and considered death from causes other than recurrence as a competing event. For this purpose, CIR was used to estimate probability of recurrence.Citation42,43 Differences in CIR across age groups and within each age category, across histologies, were assessed using Gray's method for univariate nonparametric analyses. In addition, in the octogenarians group, we examined whether risk of recurrence differed by levels of each immune marker (high vs. low). Fine-Gray's method for multivariate analysis was used to examine independent associations between variables that were significant on the univariate analysis and risk of recurrence, after adjustment for potential confounders.Citation42-44 All P-values were based on 2-tailed statistical analysis and P < 0.05 was considered indicative of statistical significance. All analyses were done using R version 3.1.1 (survival and cmprsk packages) and SAS v9.2 (SAS Institute Inc., Cary, NC, USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Joe Dycoco of the MSK Thoracic Surgery Service for his help with the Thoracic Service's Lung Cancer Database; Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance; and Irina Linkov of the MSK Department of Pathology for performing the immunohistochemical staining.
Funding
The authors’ laboratory work is supported by grants from the National Institutes of Health (R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705–06, U54 CA137788, P30 CA008748, and P50 CA086438-13), the US Department of Defense (PR101053 and LC110202), and the Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
References
- Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer 2002; 94(10):2766-92; PMID:12173348; http://dx.doi.org/10.1002/cncr.10593
- Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, Ramalingam SS. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J clin oncol 2007; 25(35):5570-7; PMID:18065729; http://dx.doi.org/10.1200/JCO.2007.12.5435
- Werner CA. The Older Population: 2010. The 2010 Census Briefs 2011.
- van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, Prokop M, Habbema JD, Oudkerk M, van Klaveren RJ. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007; 120(4):868-74; PMID:17131307; http://dx.doi.org/10.1002/ijc.22134
- National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395-409; PMID:21714641; http://dx.doi.org/10.1056/NEJMoa1102873
- National Lung Screening Trial Research Team, Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, Gareen IF, Gatsonis C, Goldin J, et al. The national lung screening trial: overview and study design. Radiology 2011; 258(1):243-53; PMID:21045183; http://dx.doi.org/10.1148/radiol.10091808
- Sacher AG, Le LW, Leighl NB, Coate LE. Elderly patients with advanced NSCLC in phase III clinical trials: are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol 2013; 8(3):366-8; PMID:23407560; http://dx.doi.org/10.1097/JTO.0b013e31827e2145
- Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J clin oncol 2012; 30(17):2036-8; PMID:22547597; http://dx.doi.org/10.1200/JCO.2012.41.6727
- Pallis AG, Ring A, Fortpied C, Penninckx B, Van Nes MC, Wedding U, Vonminckwitz G, Johnson CD, Wyld L, Timmer-Bonte A, et al. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol 2011; 22(8):1922-6; PMID:21266517; http://dx.doi.org/10.1093/annonc/mdq687
- Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol 2009; 9(1):57-62; PMID:19104499; http://dx.doi.org/10.1038/nri2471
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol 2005; 174(11):7446-52; PMID:15905594; http://dx.doi.org/10.4049/jimmunol.174.11.7446
- Pawelec G, Derhovanessian E, Larbi A. Immunosenescence and cancer. Crit Rev Oncol Hematol 2010; 75(2):165-72; PMID:20656212; http://dx.doi.org/10.1016/j.critrevonc.2010.06.012
- Ruby CE, Weinberg AD. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol 2009; 182(3):1481-9; PMID:19155495; http://dx.doi.org/10.4049/jimmunol.182.3.1481
- Provinciali M, Argentati K, Tibaldi A. Efficacy of cancer gene therapy in aging: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther 2000; 7(7):624-32; PMID:10819579; http://dx.doi.org/10.1038/sj.gt.3301131
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313(5795):1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Mlecnik B, Tosolini M, Kirilovsky A, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29(6):610-8; PMID:21245428; http://dx.doi.org/10.1200/JCO.2010.30.5425
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353(25):2654-66; PMID:16371631; http://dx.doi.org/10.1056/NEJMoa051424
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348(3):203-13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29(15):1949-55; PMID:21483002; http://dx.doi.org/10.1200/JCO.2010.30.5037
- Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, Sadelain M, Adusumilli PS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013; 31(4):490-8; PMID:23269987; http://dx.doi.org/10.1200/JCO.2012.45.2052
- Bograd AJ, Suzuki K, Vertes E, Colovos C, Morales EA, Sadelain M, Adusumilli PS. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol Immunother 2011; 60(11):1509-27; PMID:21913025; http://dx.doi.org/10.1007/s00262-011-1103-6
- Suzuki K, Kachala SS, Kadota K, Shen R, Mo Q, Beer DG, Rusch VW, Travis WD, Adusumilli PS. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res 2011; 17(16):5247-56; PMID:21659461; http://dx.doi.org/10.1158/1078-0432.CCR-10-2805
- Suzuki K, Kadota K, Sima CS, Sadelain M, Rusch VW, Travis WD, Adusumilli PS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother 2011; 60(12):1721-8; PMID:21769693; http://dx.doi.org/10.1007/s00262-011-1073-8
- Nakakubo Y, Miyamoto M, Cho Y, Hida Y, Oshikiri T, Suzuoki M, Hiraoka K, Itoh T, Kondo S, Katoh H. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer 2003; 89(9):1736-42; PMID:14583778; http://dx.doi.org/10.1038/sj.bjc.6601331
- Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci 2003; 94(11):1003-9; PMID:14611679; http://dx.doi.org/10.1111/j.1349-7006.2003.tb01392.x
- Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol 2010; 5(5):585-90; PMID:20234320
- Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009; 137(4):1270-9; PMID:19577568; http://dx.doi.org/10.1053/j.gastro.2009.06.053
- Ng R, de Boer R, Green MD. Undertreatment of elderly patients with non-small-cell lung cancer. Clin Lung Cancer 2005; 7(3):168-74; PMID:16354310; http://dx.doi.org/10.3816/CLC.2005.n.031
- Sawada S, Komori E, Nogami N, Bessho A, Segawa Y, Shinkai T, Nakata M, Yamashita M. Advanced age is not correlated with either short-term or long-term postoperative results in lung cancer patients in good clinical condition. Chest 2005; 128(3):1557-63; PMID:16162758; http://dx.doi.org/10.1378/chest.128.3.1557
- Blanchard EM, Arnaoutakis K, Hesketh PJ. Lung cancer in octogenarians. J Thorac Oncol 2010; 5(6):909-16; PMID:20521358; http://dx.doi.org/10.1097/JTO.0b013e3181d89b48
- Goodgame B, Viswanathan A, Zoole J, Gao F, Miller CR, Subramanian J, Meyers BF, Patterson AG, Govindan R. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J Thorac Oncol 2009; 4(11):1370-4; PMID:19692932; http://dx.doi.org/10.1097/JTO.0b013e3181b6bc1b
- Hanagiri T, Muranaka H, Hashimoto M, Nagashima A, Yasumoto K. Results of surgical treatment of lung cancer in octogenarians. Lung Cancer 1999; 23(2):129-33; PMID:10217616; http://dx.doi.org/10.1016/S0169-5002(99)00006-9
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004; 291(22):2720-6; PMID:15187053; http://dx.doi.org/10.1001/jama.291.22.2720
- Jennens RR, Giles GG, Fox RM. Increasing underrepresentation of elderly patients with advanced colorectal or non-small-cell lung cancer in chemotherapy trials. Intern Med J 2006; 36(4):216-20; PMID:16640737; http://dx.doi.org/10.1111/j.1445-5994.2006.01033.x
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013; 73(8):2381-8; PMID:23580578; http://dx.doi.org/10.1158/0008-5472.CAN-12-3932
- Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol 2005; 23(35):8959-67; PMID:16219934; http://dx.doi.org/10.1200/JCO.2005.01.4910
- Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC cancer 2010; 10:112; PMID:20338029; http://dx.doi.org/10.1186/1471-2407-10-112
- Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother 2012; 61(10):1849-56; PMID:22456757; http://dx.doi.org/10.1007/s00262-012-1231-7
- Rusch VW, Appelman HD., Blackstone E. AJCC Cancer Staging Manual, 7th edition edn, New York, NY: Springer; 2009.
- Sarkaria IS, Zakowski MF, Pham D, Pham D, Hezel M, Ebright MI, Chuai S, Venkatraman ES, Kris MG, Rusch VW, Singh B. Epidermal growth factor receptor signaling in adenocarcinomas with bronchioloalveolar components. Ann Thorac Surg 2008; 85(1):216-23; PMID:18154814; http://dx.doi.org/10.1016/j.athoracsur.2007.07.046
- Kadota K, Bograd A, Nitadori J, Cherkassky L, Rusch VW, Travis WD, Sadelain M, Adusumilli PS. Prognostic impact of tumor-infiltrating immune cells in lung squamous cell carcinoma. J Thorac Oncol. 2013; 8(Supple 2):S378.
- Chappell R. Competing risk analyses: how are they different and why should you care? Clin Cancer Res 2012; 18(8):2127-9; PMID:22427347; http://dx.doi.org/10.1158/1078-0432.CCR-12-0455
- Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res 2012; 18(8):2301-8; PMID:22282466; http://dx.doi.org/10.1158/1078-0432.CCR-11-2097
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16(3):1141-54; http://dx.doi.org/10.1214/aos/1176350951
