Abstract
Tuberculosis (TB) remains the world's leading cause of morbidity and mortality. Although Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the only vaccine currently in use, its efficacy is highly variable. It has been suggested that early antigenic presentation is a pivotal event leading to a better immune response in TB vaccine models. To investigate this further, we compared in vitro cell-mediated immune responses in the context of early sensitization with TB (i.e. healthy adults vaccinated with BCG when they were young, HD; n = 25) to those in its absence (i.e., newborns with naïve immunity to TB, UV; n = 10) by challenging mononuclear cells with BCG Moreau. After 48 hours, CD4+ and CD8+ T cells were harvested from both groups and stained for PD-1/CD25/ FOXP3. In addition, supernatants were assayed for a broad range of cytokines using an array system. The HD group showed robust reactivity to Protein Purified Derivative and BCG while the naïve, UV group did not. Similarly, in terms of PD-1 expression and Treg cells (CD4+/CD25high+/FOXP3+), only the HD group showed higher levels in CD4 lymphocytes. Otherwise, only the UV group showed expression of CD25dim+ as an activation marker dependent on BCG infection. In terms of cytokines, the HD group showed higher levels of Th1 (IL-2/TNF-α/IFN-γ) and regulatory (IL-10) profiles, with monocytes, but not Tr1 cells, acting as the main source of IL-10. Taken together, our results highlight critical roles of early sensitization with TB in mounting cell-mediated immune responses.
Abbreviations:
- TB, tuberculosis
- BCG, bacillus calmette-guérin
- HD, healthy donor
- UV, umbilical vein
- PBMC, peripheral blood mononuclear cells
- CBMC, cord blood mononuclear cells
- ELISPOT, enzyme linked immunospot
- ELISA, enzyme-linked immunosorbent assay
- PPD, protein purified derivative
- PHA, phytohaemaglutinin
- FACS, fluorescence activating cell sorting
- CBA, cytometric beads array kit
- iNKT, invariant natural killer T cells
- HLA, human leukocyte antigen
- HIV, human immunodeficiency virus
Introduction
Tuberculosis (TB) still remains a serious global health problem, with a total of 8.8 million new cases and 3 million deaths each year.Citation1 Current projections point toward one third of the world population being infected with Mycobacterium tuberculosis and 5–10% of those will develop the disease during their life-time.Citation2
The primary TB vaccine is M. bovis bacille Calmette-Guérin (BCG), which has been employed for almost a century to stop the disease. Although BCG is the most widely used vaccine globally, its effectiveness remains controversial. Epidemiological studies and reviews imply BCG as affording systemic protection against mostly the meningeal and miliary clinical forms of TB in children than against the pulmonary clinical form in adults.Citation3,4 In endemic settings circulated along the equator and tropics regions, such as Brazil, the vaccine has provided unpredictable results.Citation4 Consequently, further studies are needed to better understand how BCG confers protection in humans.Citation5
Our preceding studies support the premise that BCG induces distinct cell-death patterns during the maturation of the immune system.Citation6,7 In the study, we observed increasing apoptosis events in BCG-stimulated monocytes from healthy, vaccinated adults, associated with a release of IL-1β and TNF-α, but not metalloproteinase-9. Conversely, higher monocytes necrosis, but not apoptosis, was observed following the infection of umbilical vein cells from naïve, neonates. This pattern was paralleled by different pro-inflammatory cytokine levels when compared to adults. However, a key limitation of the study was that different subpopulations of white blood cells (i.e. lymphocytes) after BCG infection were not probed further.
To address remaining critical issues related to the adaptive cellular immune responses to BCG, we have used the same model of infecting human cells with BCG Moreau for 48 hours and characterized both T cell phenotypes and secreted soluble factors in the context of presence/absence of BCG sensitization (i.e., vaccinated adults vs. naïve neonates). By this way, there is an input for better understanding of the protective factors afforded by BCG against TB that would help to identify the processes by which this protection is achieved, thus opening up a horizon for its future improved clinical applicability.
Studies to date have not established any relationship in the context of in vitro cell-mediated immune responses comparing BCG Moreau-vaccinated adults vs. non-BCG Moreau vaccinated neonates. This is a community based cross-sectional population study of a sample from Brazil, and the rationale is to uncover critical aspects of CD4+ and CD8+ T cells and their cytokines in inducing either an inflammatory or regulatory profile.
Results
In vitro T-cell immune response against BCG Moreau vaccine using a modified IFN-gamma ELISPOT assay
To identify mechanisms that may confer protection against M. tuberculosis, cell-mediated immune responses in humans were investigated in vitro in individuals with and without prior exposures to BCG. To this end, we enrolled 2 groups of donors: Healthy donor adults (HD) who have been already vaccinated with BCG Moreau during childhood (BCG vaccination in Brazil is mandatory after birth), and naïve individuals (i.e. newborns) who have never been exposed to mycobacteria. For the naïve individuals (UV), their umbilical vein mononuclear cells (CBMC) were promptly collected after their delivery.
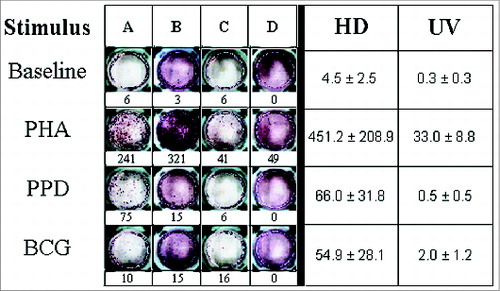
Images and frequencies of IFN-γ enzyme linked immunospot (ELISPOT) responses at the single-cell level for cell media (Baseline), PHA, PPD and M. bovis BCG tested in the HD and UV groups are shown in . As controls, positive responses (P-value ≤ 0.02) to PHA, and negative ones to cell media were observed in all individuals. Remarkably, the UV group (n=4) showed no response to all mycobacteria-related stimuli tested (). In contrast, the HD group (n = 11) showed robust reactivity (P-value ≤ 0.01) to the same stimuli, except to the M. bovis HSP65 recombinant antigen (6.3 ± 1.4, p-value = n.s.). The HSP65 antigen was not tested in the UV group.
Figure 1. IFN-γ production in primary mononuclear cell cultures challenged with Phytohaemaglutinin (PHA), Purified Protein Derivative (PPD) and M. bovis BCG. In the left panel, ELISPOT assay results for representative healthy adult mononuclear (A and B) and neonate cord blood cells (C and D) are shown. The corresponding production levels in terms of counts of spot forming colonies are described below. PHA, PPD and BCG antigens were used at concentrations of 1% and 5 μg/ml, respectively. In the right panel, the average productions (± SEM) for healthy adult mononuclear (HD) and neonate cord blood cells (UV) are shown.
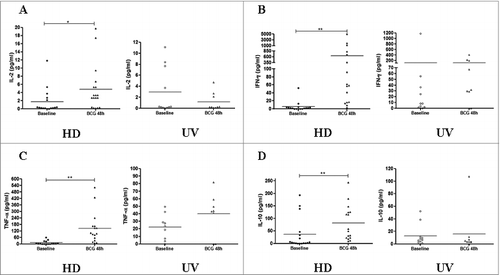
Cytokines signature profiling for the in vitro T-cell immune response against BCG Moreau vaccine
The production of IFN-γ and TNF-α are associated with effective T cell-mediated immunity against M. tuberculosis, and the assessment of cytokine productions is an important component of studies of cellular immune responses to successful vaccination.Citation4 Overall, the magnitude of the in vitro immune responses for the 6 cytokines tested, i.e., IL-2, IL-10, TNF-α and IFN-γ (), as well as IL-4 and IL-6 (data not shown), in the CBMC after BCG infection, did not increase significantly. In contrast, IL-2, IFN-γ, TNF-α and IL-10 levels increased significantly at 48 hours of BCG infection in the HD group (). Additional analyses were performed (1) to quantify the ratio of IFN-γ/IL-10 in the Multiplex assay in samples from both groups, and (2) to compare the IFN-γ production between ELISPOT and Multiplex array kit for the same adult individuals tested in parallel. Not surprisingly, M. bovis BCG induced a significantly elevated IFN-γ/IL-10 ratio only in the HD group (p-value < 0.01), confirming a polarized Th1 pattern in vitro (). On the other hand, due to uneven range related to intensity of responses, IFN-γ levels in the HD group had no direct correlation between methods (data not shown).
Phenotyping assays by FACS uncovering the in vitro T-cell immune response against BCG Moreau vaccine
It has been shown that the cross-talk between different immune cells leading to antigen-presenting cell maturation and lymphocyte activation was found to be multi-directional, involving not only soluble factors release (as shown above) but also cell-to-cell contacts, and the final outcome of these cellular interactions may have a dramatic impact on the quality and strength of the downstream immune responses, mainly in the context of early responses to infectious agents.Citation8 In our previous work, we showed that presence/absence of early exposure to TB can drastically influence the patterns of BCG-induced monocyte cell death.Citation6 To investigate this further, we performed phenotyping assays in 48 hours-BCG infected cultures focusing on the autologous lymphocytes to detect Treg cells that are activated and express PD-1. summarizes all phenotypic findings.
Figure 3. Treg cells, PD-1 and CD25-activation markers induced by BCG Moreau at 48h in T cells from healthy donor (HD) and umbilical vein (UV) individuals. Bars depict the mean levels in each condition. *P ≤ 0.05; **P < 0.01. Insert shows a typical profile from one representative experiment for PD-1 and CD4 double staining for (A) baseline and (B) BCG Moreau-infected PBMC cultures evaluated by flow cytometry. In the dot plot, percentages of cells are indicated in each quadrant. The viable lymphocyte population was previously gated on the basis of their light scattering properties. FL-1 and FL-8 represent CD4 FITC and PD-1 APC staining, respectively.
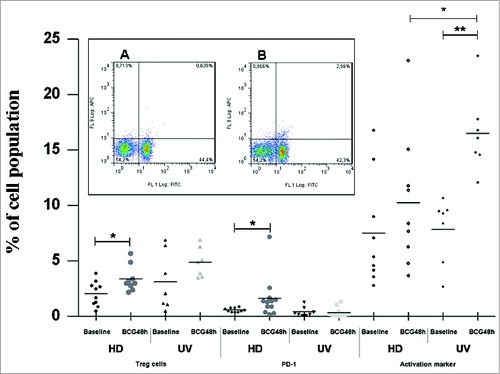
In , the HD group shows a higher percentage of Treg cells (CD25high+ and FOXP3+ co-expression) in the CD4+ compartment after BCG infection than the UV group (P-value < 0.05). Since T lymphocytes expressing PD-1 are considered to be exhausted,Citation9 the expression levels of PD-1 on CD4+ () and CD8+ T cells were also determined (0.5 ± 0.1 and 0.9 ± 0.2; 1.2 ± 0.4 and 2.1 ± 0.7, at baseline and BCG-infected for the HD and UV groups, respectively. p-value = n.s.). Thus, the HD group showed a higher percentage of CD4+ T cells expressing PD-1 after BCG infection (p-value < 0.05) than the UV group. On the other hand, high level of the activation marker (CD25dim+) expressing-CD4+ T cells was observed in the UV group after BCG infection (p-value < 0.01), but not in the HD group. The lymphocyte subset has long been recognized in human neonatal CBMC as invariant natural killer T cells (iNKT) constitutively expressing CD25 and CD4+ markers.Citation10,11 It seems possible that a lipid or glycolipid moiety expressed by BCG Moreau and presented by CD1d to iNKT cells contributed to the enhanced levels (p-value < 0.01) found after infection, since no difference was observed when comparing baseline levels between the 2 groups. In favor of this hypothesis, during BCG infection, the UV group showed higher levels of CD25dim+ expression than the HD group (p-value < 0.05). Of note, PD-1 expressing CD4+ T cells in either group had no direct correlation with activated, CD25dim+/CD4+ T cells (data not shown), in an assumption of unique cell population and in line with other studies.Citation9
The in vitro T-cell profile induced by BCG Moreau vaccine in healthy donors is neither Th3, nor Tr1
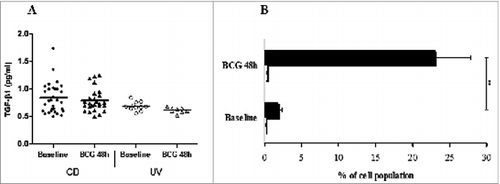
The inhibitory cytokines TGF-β and IL-10 have been implicated in CD4+FOXP3+ induced (adaptive) regulatory T (iTreg) cells function. In order to indirectly identify whether that Treg cell population described in the has a Th3 cell phenotype after BCG infection in the HD group, we performed ELISA for TGF-β in the supernatants of stimulated cultures, and those levels did not increase significantly in either tested group (). Similarly, we also performed additional FACS analysis to ultimately identify whether a Tr1-like phenotype was induced after BCG infection in the HD group, and results show that monocytes, but not Tr1 cells, are the main source of IL-10 in the PBMC in our system ().
Figure 4. (A) Transforming growth factor (TGF)-β1 levels in pg/mL measured by ELISA in the supernatants of PBMCs from healthy donor (HD) and CBMCs from umbilical vein (UV) individuals stimulated with BCG Moreau for 48h. Horizontal bars represent mean values in each condition. (B) Tr1 cells (CD4+IL-10+FOXP3+; open bars) and monocytes+IL-10+ (black bars) induced by BCG Moreau at 48 h in PBMC from healthy donor. Bars depict the mean levels (+ SEM) in each condition. **P < 0.01.
Discussion
We hypothesized that BCG induces immune response patterns that are dependent on the developmental stage of the immune system, and that subsequent anti-mycobacterial immune responses may be profoundly affected. To this end, we have presented results on in vitro T cell-profiles induced by the BCG Moreau vaccine in 2 cohorts distinguished by their BCG sensitization: already sensitized adults (either by vaccination after birth or prior exposure to environmental mycobacteria), and neonates with naïve immune response against mycobacterial antigens. In support of this hypothesis, the 2 groups showed strikingly different T-cell profiles. The BCG-sensitized group showed increased levels of Treg cell markers which include IL-10, prototypic Th1 cell profile resembling the polyfunctional T cells, and PD-1 expression on CD4+ T cells increased. In contrast, only the BCG-naive group showed a single activated profile in non-primed lymphocytes. Furthermore, the BCG-sensitized group showed roughly 14-fold higher levels of immune responses to PHA when compared to the BCG-naive group. The ELISPOT data provided evidences that in vitro Th1-immune response of neonates was deficient when cells were in contact with recombinant antigens, except for a positive immune response against the mitogen.Citation7
Our IFN-gamma release assay results are notable since this has not been done using umbilical vein cells from naïve individuals before. Nevertheless, a study by Watkins and colleagues (2008) has shown productions of IFN-γ, IL-10, IL-12, IL-5 and IL-13, but no IL-4, in a maximum of 6 days-stimulated CBMC cultures. In the study, however, the NK cell population, instead of T cells, was responsible for IFN-γ specific production.
The reasons behind results for the neonate group are speculative: perhaps due to a higher amount of circulating immature immune cells or to a lack of exposure to mycobacterial antigens. Actually, because of decreased Th1-cell-associated cytokines production found by othersCitation13 and also in the current study employing pathogen-related antigens, it is thought that the neonatal innate immune system is generally weakened or depressed. In addition, the bias against Th1-cell-polarizing cytokines contributes to impairment of neonatal immune responses to most vaccines.Citation14 Furthermore, there is an age-dependent maturation of the immune response.
However, the previous assertion that the neonatal immune system is partly down-regulated might not be entirely true, since high levels of CD25dim+ induced in M. bovis-naive CD4+ T cells were observed here. Actually, human neonatal iNKT constitutively express CD25, and display 2 main CD4 subsets.Citation10,11 The CD4+ iNKT cells dominate in fetal and neonatal blood (> 90% iNKT population), and often suggests that intrinsic expression might mirror a distinct developmental stage that plays a key role in immunity in early life. Neonatal iNKT cells have an inherent and substantially reduced proliferation threshold, and there is a recent suggestion for a potential role of CD25dim+ expression in ensuring survival, stability and expansion of a structurally diverse antigenic receptor iNKT-cell repertoire in early stages.Citation15 However, there is a unifying belief that neonatal CD25+ iNKT cells are not simply activated cells, but rather represent a developmentally distinct subset, and is also supported by the absence of other markers of recent T-cell activation, including CD69 and HLA-DR,Citation16 which remain to be further evaluated in our system.
Polyfunctional T cells have been originally characterized as high IL-2, TNF-α and IFN-γ producers.Citation17 As previously stated, identical cytokine profiles were detected in our BCG-infected PBMC cultures, which make attractive the hypothesis that BCG Moreau vaccine also induces that phenotype in vitro. In support of this hypothesis, data from South Africa and Australia studies demonstrated increased levels of polyfunctional T cells generation.Citation18,19
Treg cells represent well-characterized CD4+ T lymphocyte subsets bearing a canonical high CD25 expression plus the intracellular FOXP3 nuclear factor. Suppression of M. tuberculosis specific T cells by blocking their effector function is the major aspect of Tregs, and they are important for controlling the immune response in TB (Reviewed byCitation20). Recently, Reiley and colleagues (2010) speculated that exposure to environmental mycobacteria may stimulate pathogen-specific Treg cells, cross-reacting with BCG antigens and hampering vaccine-induced immune response against the bacteria. In fact, there is a strong evidence to support the hypothesis that Treg cells induced by environmental mycobacteria restrict the immunity afforded by BCG vaccine. Therefore, Treg cells, rather than Th2 cells induced by environmental mycobacteria,Citation22 can suppress inflammatory responses to BCG. In another study, M. chelonae-sensitized mice produced excessive Tr1 cells responses with high IL-10 levels after challenge with BCG, indicating that environmental mycobacteria can support expansion or recruitment of Treg cells, which again affects the immune response to BCG.Citation23 Also, we and others have shown that Treg cell levels increased after BCG infection in sensitized adults.Citation24,25 Alternatively, and together with the increased IL-10 levels found exclusively when PBMCs were infected with BCG Moreau in adults, it suggests that a potential regulatory profile was indeed in vitro induced. In fact, peripheral blood CD14+ monocytes-derived DCs are significant sources of IL-10 as a driving force to a more immunoregulatory phenotype,Citation26,27 and thus compelling data generated in the present study ruled out a Tr1-like phenotype. Rather, monocytes were found to be the major source of IL-10 in BCG-infected PBMC cultures from healthy sensitized adults. Likewise, no Th3 cell phenotype was evident after BCG infection in the HD group: Equivalent TGF-β levels were found regardless of the stimulus employed.
However, albeit there is a trend for similarly high levels, we did not observe a significant Treg cells level directly induced by BCG in naïve, neonate individuals. We hypothesize that once neonate individuals are exposed to environmental mycobacteria, levels of Treg cells after infection with BCG will increase. Thus, our current working model is that protective immunity induced by BCG vaccination is compromised, because antigen-specific Treg cells cross-react between environmental mycobacteria and the BCG vaccine. This cross-reaction in turn may suppress the Th2 cells development and compromise protective immunity induced by BCG vaccination.Citation27
T cell exhaustion develops under conditions of antigen persistence caused by infection with various chronic pathogens, such as HIV and M. tuberculosis.Citation9 Thus, PD-1-expressing T lymphocytes in humans have gained much importance recently in HIV research as playing a role as intrinsic factors of inhibitory effect on the proliferation and production of cytokines.Citation28 Initial characterization of the PD-1-PD-L1/L2 pathway, members of the CD28 family of receptors, has revealed down-regulation in TCR- and CD28-mediated signals,Citation28 as well as CD25 in CD8+ T cells.Citation9 These signaling events collectively modulate immune responses, presumably by inhibiting cell cycle progression. In the present study, although the expression was first described for HIV-specific CD8+ T cells associated with exhaustion and disease progression in infected patients,Citation28 we found higher PD-1 levels induced by BCG only in CD4+ T cells from adults, suggesting that the molecule plays a critical role in modulating immune responses in the BCG-sensitized group. It remains to be determined further the co-expression of additional inhibitory receptors, such as CTLA-4, as a confirmatory approach. Multiple inhibitory receptors independently contribute to T cell exhaustion during chronic infection, suggesting that its reversal could be achieved by therapeutic targeting of those pathways simultaneously.Citation9 When putting together the phenotypic data generated in our adult cohort, and while it is still not quite clear whether Treg cells induced under antigen persistent environment may directly exhaust T cells, accumulating evidence suggest that Treg cells may contribute to the reduction of T cell efficiency and secondary T cell exhaustion.Citation9
There were limitations in the current study. First, any additional analysis about demographic parameters of the cohort is incomplete due to the hospital's depository guidelines regarding blood donation. However, we have restricted the HD cohort to include only adults (≥18-years old). Second, we were unable to directly assess functional (suppressive) in vitro responses because the methodology used to characterize Treg cell responses was incomplete. Given the above, one should consider the results of this study-particularly those from the second and third approach, hypothesis-generating rather than hypothesis-confirming. Finally, due to the BCG vaccination policy in Brazil (lasting 70 years),Citation29 we could not enroll unvaccinated subjects, i.e. healthy donor adults who have never been vaccinated with BCG Moreau, to perform additional comparisons.
Taken together, the important topic of the triggering of innate and adaptive immune responses by BCG vaccines deserves further study. A better understanding of the factors that protect against TB helps us identify the processes by which this protection is achieved, thus opening up a horizon for its future clinical applicability. Ultimately, this leads to the development of improved vaccines for TB that, together with infectious diseases in general, disproportionately affects poor and marginalized populations.
Patients and Methods
Study participants
Between November 2010 and August 2012, 2 groups of donors were enrolled for this study at the Gaffrée Guinle State University Hospital of Rio de Janeiro: Healthy donor adults (HD; n=25) from the blood bank (anonymous donation policy, but included individuals age ≥18-years old), and healthy mothers who participated in puncturing umbilical cords procedures for sampling blood from newborns’ umbilical vein (UV; n=10). Exclusion criteria for those individuals utilized HIV-seronegative status, a negative history of malignant, degenerative, or transmitted diseases, diabetes mellitus, and use of corticosteroids or other immunosuppressive agents at the time of the study. This study was approved by the Institutional Review Board, and all study participants provided written informed consent as described elsewhere.Citation6
Mononuclear cells purification, cell culturing and in vitro infection with BCG
Peripheral Blood Mononuclear Cells (PBMC) and Cord Blood Mononuclear Cells (CBMC) were separated (> 92% purity) within 24 hours of obtaining the blood specimens from all study participants using a Ficoll density gradient. The collected cells were first washed 3-fold with endotoxin-free phosphate buffered saline (PBS 50 mM, pH 7.2), then suspended in DMEM medium (Sigma Immunochemicals, MD, USA) supplemented with 20% autologous serum. Cell cultures (1 × 106) were kept at 37°C in a humidified 5% CO2 atmosphere in individual 12 × 75 mm sterile polystyrene tubes (Falcon, Corning Inc., NY, USA). Previous experiments with these tubes showed a better viability of cells when compared to conventional culture plates (data not shown). The PBMC and CBMC were infected with BCG Moreau strain for a total of 48 hours as previously described.Citation6 For each participant, one vial of fresh cells (maximum of 1 × 107) and another for supernatant were kept frozen in a liquid nitrogen tank and at −70 °C, respectively.
IFN-gamma ELISPOT assay
PBMC and CBMC were thawed, and the IFN-γ ELISPOT was assayed as recommended by the T.SPOT-TB® manufacturer (Oxford Immunotech Inc., Oxford, UK) using a slightly modified protocol where original panels A and B were replaced by adding 5.0 μg/ml of either Protein Purified Derivative (PPD RT-23, Statens Serum Institut, Copenhagen, Denmark) or M. bovis HSP65 (Kindly provided by Drs. T. Ottenhoff, LUMC, The Netherlands, and E. Sampaio, FIOCRUZ, Brazil). The mitogen Phytohaemaglutinin (PHA) was used at concentration of 1%. Either cell population was never tested for original antigens from this kit. BCG Moreau at the same concentration as before, i.e., multiplicity of infection (MOI) ratio of 2:1 (bacilli:mononuclear cell ratio), was used for infection as well.Citation6,7 Once a reactive sample was developed and quantified using an image analysis software (KS-ELISPOT reader, Zeiss, Germany), it was considered positive if at least 6 spots were formed.
Phenotyping and Cytometric Beads Array (CBA) assays by Fluorescence Activating Cell Sorting (FACS)
The detection of regulatory (Treg: CD4+/CD25high+/ FOXP3+; Tr1: CD4+/IL-10+/FOXP3+), activated (CD25dim+) and PD-1+ expressing T cells were assayed in 48 hours-BCG infected PBMC and CBMC (except Tr1) by using FITC-labeled-anti-hCD4 or hCD8 plus either a Treg-staining kit (eBioscience, San Diego, CA, USA), PE-labeled-anti-hIL-10, PE-labeled-anti-hCD25 or APC-labeled-anti-hPD-1 in cold PBS plus 1% BSA. In addition, monocytes with an immunoregulatory phenotype (CD14+/IL-10+) were also assayed in some experiments of 48 hours-BCG infected PBMC by using FITC-labeled-anti-hCD14 plus a PE-labeled-anti-hIL-10. The mixtures were washed, followed by 1% paraformaldehyde. Labeled cells were run on a CyAn ADP FACS device (Beckman-Coulter/Dako, Brea, CA, USA) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA). A cell gate region was drawn around lymphocytes and monocytes to exclude debris, and a total of 10,000 events per sample were collected. Optimal gating strategies for determining Tr1 cells and monocytes were applied (Supplementary ). Thresholds and statistical markers were set for positivity by means of negative, baseline match controls as a reference. All data were expressed as the percentage of double stained-positive bright cells.
Cytokine levels were determined using the commercially available Cytometric Beads Array kit (CBA, BD Biosciences PharMingen, USA) to quantify human IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ. The CBA immunoassay in thawed supernatants was carried out according to the manufacturer's instructions.
TGF-β1 detection by Enzyme-Linked Immunosorbent Assay (ELISA)
Transforming growth factor (TGF)-β1 levels were determined using a commercially available kit (DuoSet R&D, Minneapolis, MN, USA) carried out according to the manufacturer instructions. Samples of TGF-β were activated using 1.0 N HCl and neutralized by 1.2 N NaOH/0.5 M HEPES as recommended by the kit manufacturer before being applied to the ELISA plate. The optical density was determined directly using a microplate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA) set at dual wavelengths of 450 nm and 540 nm. The 540-nm reading was subtracted from the 450-nm reading. By means of a computer-generated 4-parameter logistic (4-PL) curve fit from the standard absorbance values and the corresponding concentrations, the unknown sample concentrations were immediately calculated by the spectrophotometer software. The detection limit was 20 pg/ml.
Statistical evaluation
Data were analyzed using GraphPad Instat software. Significance of the difference in levels of immune responses between 2 groups of individuals was calculated using Student's t-test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplementary_Figure_2.bmp
Download (766.9 KB)Supplementary_Figure_1.bmp
Download (1 MB)Acknowledgments
The authors are grateful to Drs. Yohan Kim at La Jolla Institute for Allergy and Immunology (LIAI, USA) and Stuart Krassner at University of California, Irvine (UCI, USA) for text editing. We also thank the Gaffrée Guinle State University Hospital staff for their help during the clinical procedures and Dr. Cecilia Lindestam Arlehamn at LIAI for ELISPOT plate readings.
Funding
This work was partly supported by Oxford Immunotech Inc. (UK), IOC / FIOCRUZ; LP and JS are the recipients of FAPERJ scholarships, TP is the recipient of PEC / FIOCRUZ scholarship, and PRZA is granted with a CNPq research fellowship (PQ-2).
References
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet 2003; 362(9387):887-99; PMID:13678977; http://dx.doi.org/10.1016/S0140-6736(03)14333-4
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement and Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country: WHO Global Surveillance and Monitoring Project. JAMA 1999; 282:677-86; PMID:10517722; http://dx.doi.org/10.1001/jama.282.7.677
- Ponnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, Jenkins PA, Lucas SB, Liomba NG, Bliss L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 1992; 339(8794):636-9; PMID:1347338; http://dx.doi.org/10.1016/0140-6736(92)90794-4
- Antas PR, Castello-Branco LR. New vaccines against tuberculosis: lessons learned from BCG immunisation in Brazil. Trans R Soc Trop Med Hyg 2008; 102(7):628-30; PMID:18440575; http://dx.doi.org/10.1016/j.trstmh.2008.03.014
- Orme IM. The immune response to the cell wall of Mycobacterium bovis BCG. Clin Exp Immunol 1988; 71(3):388-93; PMID:3289800
- Simas CJ, Silva DP, Ponte CG, Castello-Branco LR, Antas PR. Patterns of in vitro cell-death, metaloproteinase-9 and pro-inflammatory cytokines in human monocytes induced by the BCG vaccine, Moreau strain. Vaccine 2011; 29(38):6446-50; PMID:21745518; http://dx.doi.org/10.1016/j.vaccine.2011.06.093
- Ponte CG, Antas PR, Peres LM, Marinho SP. Comments on the neonatal bacillus Calmette-Guerin vaccination: adding notes in proof of nonspecific effect. Am J Respir Crit Care Med 2013; 187(7):778-9; PMID:23540882
- Reschner A, Hubert P, Delvenne P, Boniver J, Jacobs N. Innate lymphocyte and dendritic cell cross-talk: a key factor in the regulation of the immune response. Clin Exp Immunol 2008; 152(2):219-26; PMID:18336590; http://dx.doi.org/10.1111/j.1365-2249.2008.03624.x
- Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep 2011; 44(4):217-31; PMID:21524346; http://dx.doi.org/10.5483/BMBRep.2011.44.4.217
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med 2002; 195(5):625-36; PMID:11877485; http://dx.doi.org/10.1084/jem.20011786
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med 2002; 195(5):637-41; PMID:11877486; http://dx.doi.org/10.1084/jem.20011908
- Watkins ML, Semple PL, Abel B, Hanekom WA, Kaplan G, Ress SR. Exposure of cord blood to Mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin Vaccine Immunol 2008; 15(11):1666-73; PMID:18815231; http://dx.doi.org/10.1128/CVI.00202-08
- Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod 2001; 16(10):2219-26; PMID:11574519; http://dx.doi.org/10.1093/humrep/16.10.2219
- Siegrist CA. Vaccination in the neonatal period and early infancy. Int Rev Immunol 2000; 19:195-219; PMID:10763709; http://dx.doi.org/10.3109/08830180009088505
- Ladd M, Sharma A, Huang Q, Wang AY, Xu L, Genowati I, Levings MK, Lavoie PM. Natural killer T cells constitutively expressing the interleukin-2 receptor alpha chain early in life are primed to respond to lower antigenic stimulation. Immunology 2010; 131(2):289-99; PMID:20545784; http://dx.doi.org/10.1111/j.1365-2567.2010.03304.x
- Eger KA, Sundrud MS, Motsinger AA, Tseng M, Van KL, Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PLoS ONE 2006;1:e50; PMID:17183680; http://dx.doi.org/10.1371/journal.pone.0000050
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13(7):843-50; PMID:17558415; http://dx.doi.org/10.1038/nm1592
- Kagina BM, Abel B, Bowmaker M, Scriba TJ, Gelderbloem S, Smit E, Erasmus M, Nene N, Walzl G, Black G, et al. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine 2009; 27(40):5488-95; PMID:19616494; http://dx.doi.org/10.1016/j.vaccine.2009.06.103
- Ritz N, Dutta B, Donath S, Casalaz D, Connell TG, Tebruegge M, Robins-Browne R, Hanekom WA, Britton WJ, Curtis N. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med 2012; 185(2):213-22; PMID:22071384; http://dx.doi.org/10.1164/rccm.201104-0714OC
- Fletcher HA. Correlates of immune protection from tuberculosis. Curr Mol Med 2007; 7(3):319-25; PMID:17504116; http://dx.doi.org/10.2174/156652407780598520
- Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 2010; 107(45):19408-13; PMID:20962277; http://dx.doi.org/10.1073/pnas.1006298107
- Gooding TM, Johnson PD, Smith M, Kemp AS, Robins-Browne RM. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect Immun 2002; 70(10):5562-7; PMID:12228283; http://dx.doi.org/10.1128/IAI.70.10.5562-5567.2002
- Ho P, Wei X, Seah GT. Regulatory T cells induced by Mycobacterium chelonae sensitization influence murine responses to bacille Calmette-Guerin. J Leukoc Biol 2010; 88(6):1073-80; PMID:20651297; http://dx.doi.org/10.1189/jlb.0809582
- Antas PR, Sampaio EP. Another round for the CD4+CD25+ regulatory T cells in patients with tuberculosis. Am J Respir Crit Care Med 2007; 176(2):214-5; PMID:17617535; http://dx.doi.org/10.1164/ajrccm.176.2.214
- Li L, Lao SH, Wu CY. Increased frequency of CD4(+)CD25(high) Treg cells inhibit BCG-specific induction of IFN-gamma by CD4(+) T cells from TB patients. Tuberculosis 2007; 87(6):526-34; PMID:17851131; http://dx.doi.org/10.1016/j.tube.2007.07.004
- Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology 2005; 114(2):204-12; PMID:15667565; http://dx.doi.org/10.1111/j.1365-2567.2004.02076.x
- Coleman MM, Keane J, Mills KH. Editorial: Tregs and BCG-dangerous liaisons in TB. J Leukoc Biol 2010; 88(6):1067-9; PMID:21123295; http://dx.doi.org/10.1189/jlb.0710419
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443(7109):350-4; PMID:16921384: http://dx.doi.org/10.1038/nature05115
- Benevolo-de-Andrade TC, Monteiro-Maia R, Cosgrove C, Castello-Branco LR. BCG Moreau Rio de Janeiro: an oral vaccine against tuberculosis-review. Mem Inst Oswaldo Cruz 2005; 100(5):459-65; PMID:16184220; http://dx.doi.org/10.1590/S0074-02762005000500002