Abstract
This updated systematic review and meta-analyses aims to systematically evaluate the cross-protection of seasonal influenza vaccines against the 2009 pandemic A (H1N1) influenza infection, and investigate the potential effect of the influenza strains circulating previous to the pandemic on the association between vaccine receipt and pandemic infection. In addition, subgroup analysis was performed based on the study locations and previous circulating influenza viruses. Relevant articles in English and Chinese from 2009 to October 2013 were systematically searched, and 21 eligible studies were included. For case-control studies, an insignificant 20% reduced risk for pandemic influenza infection based on combined national data (OR = 0.80; 95%CI: 0.60, 1.05) was calculated for people receiving seasonal influenza vaccination. However, for RCTs, an insignificant increase in the risk of seasonal influenza vaccines was observed (RR = 1.27; 95% CI: 0.46, 3.53). For the subgroup analysis, a significant 35% cross-protection was observed in the subgroup where influenza A outbreaks were detected before the 2009 pandemic. Moreover, the results indicated that seasonal influenza vaccination may reduce the risk of influenza-like illnesses (ILIs) (RR = 0.91; 95% CI: 0.84, 0.99). Our findings partially support the hypothesis that seasonal vaccines may offer moderate cross-protection for adults against laboratory-confirmed pandemic influenza A (H1N1) infection and ILIs. Further immunological studies are needed to understand the mechanism underlying these findings.
Background
Influenza is a common infectious disease that significantly affects public health because of its high prevalence and mortality. In April 2009, a novel influenza A (H1N1) virus emerged in Mexico and the USA; this virus rapidly spread worldwide and caused the first influenza pandemic of the 21st century.Citation1 This novel H1N1 virus was found to be antigenically related to the subtypes circulating in pigs and has a hemagglutinin and neuraminidase subtype different from those that are circulating in humans.Citation2,3 More than 200 countries and areas have been affected and over 18000 people have died during the pandemic.Citation4
Several studiesCitation5-12 have reported on the protective effect of seasonal influenza vaccine against the 2009 pandemic influenza infection. Although some reports presented significant results,Citation5-10 the cross-protection of seasonal influenza vaccine remains controversial. Several other studies had studied this issue, but with mixed results.Citation13-19 Prior meta-analysesCitation20 from RCTs or cohort studies cannot provide sufficient data. Thus, results of previous studies were inadequate to draw a definitive conclusion on this issue. Since then, further studies have been published, which prompted us to conduct this systematic review and meta-analyses to determine whether seasonal influenza vaccination can affect the prevention of the 2009 pandemic A (H1N1) influenza infection. Compared with our prior meta-analysis, this review includes 6 more studies. Moreover, previous experiments on ferret have shown that seasonal influenza infection could protect humans against this pandemic infection.Citation17 Thus, a subgroup analysis was conducted to investigate further the effect of previous influenza outbreaks on the association between seasonal influenza vaccination and 2009 pandemic infection.
Vaccination has been the most important intervention to prevent and control the epidemic and pandemic influenza for over a half century.Citation22 Given the difficulty in predicting the next pandemic influenza strain and when the pandemic will occur, we should consider the potential effect of seasonal vaccination on the early stage of an influenza pandemic. Thus, more evidence should be provided to arrive at a better conclusion on the relationship between an influenza pandemic and seasonal influenza vaccines.
Results
General information
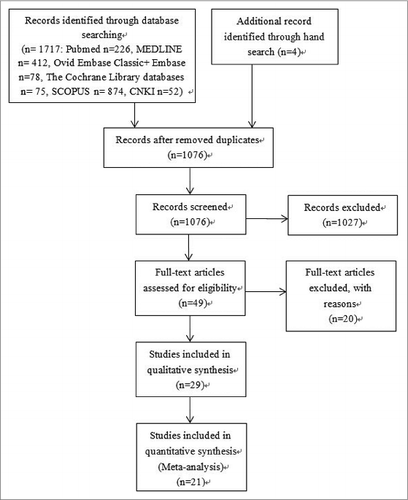
presents the flow diagram of the study selection procedure. Overall, 1721 studies were identified, and 28 eligible studies were included in our systematic review after screening. The characteristics of the 28 included studies, which address the cross-protection of seasonal influenza vaccine against 2009 pandemic influenza A (H1N1) infection, are summarized in Supplemental Tables 1-4. A total of 135,347 participants aged 0 to over 75 years were included. Most studies demonstrated a roughly balanced gender ratio among the participants, except for the case-control study conducted on the military.Citation6 In this review, we divided the study by Skowronski et al.Citation15 into one cohort study and 3 case-control studies (Quebec household transmission study as Skowronski-a, national sentinel monitoring system as Skowronski-b, Quebec population case-control study as Skowronski-c, Ontario test-negative case-control study as Skowronski-d). This systematic review included 4 RCTs,Citation11,12,18,19 4 cohort studies,Citation15,16,22,23 and 20 case-control studiesCitation5-10,Citation13-15,Citation24-32. In addition, 2 surveillance reports and one outbreak investigation were included. Considerable variations were observed among the studies in terms of the study size, age of participants, study locations, and study outcomes.
Figure 1. Trial identification flowchart for English and Chinese databases: Adapted from PRISMA 2009 flow chart.Citation22
The four RCTs were considered as higher quality studies according to Jadad score. Moreover, no underlying high risk of bias was detected. For the non-randomized studies, based on the NOS scoring system, 3 of the cohort studies had moderate risk, whereas one studyCitation22 had high risk of bias. For the case-control studies, 9 had low-risk biasCitation8,9,10,14,15,24,28,29,31, while the other 11 had moderate-risk biasCitation5-7,Citation13,15,Citation25-27,Citation30,32. The details of the quality assessment are shown in the Supplemental Tables 5-8.
Meta-analyses
The combined effects of seasonal influenza vaccination on pandemic influenza laboratory-confirmed cases and ILIs were interpreted and analyzed through forest plots.
Randomized controlled trials (RCTs)
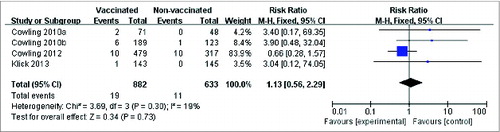
For the pooled analysis of seasonal influenza vaccine against laboratory-confirmed pandemic infection, an insignificant relative risk increase of 27% was calculated (RR = 1.27; 95% CI: 0.46, 3.53; P = 0.64), and no significant heterogeneity was observed (I2 = 19%; P = 0.30) (). In addition, the cross-protection by the seasonal vaccine on ILIs has also been reported in RCTs. A statistically significant risk reduction was detected between the vaccinated and the control groups (RR = 0.91; 95% CI: 0.84, 0.99; P = 0.02) with no evidence of statistical heterogeneity (I2 = 0%; P = 0.52) (Fig. S1).
Cohort study
Two cohort studiesCitation15-16 reported the vaccine effect on laboratory-confirmed cases and 2 studies have reported about ILIs. All studies provided insignificant results (Figs. S2 and S3).
Case-control study
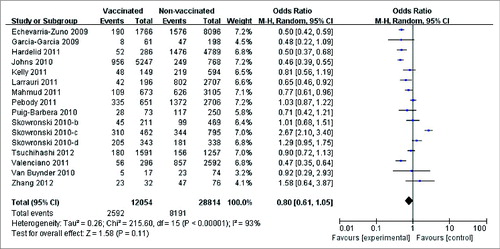
A slight risk reduction was observed in 16 case-control studies Citation5-7,Citation9,10,Citation13-15,Citation25,27,Citation29-32 that reported laboratory-confirmed cases (OR: 0.80; 95% CI: 0.61, 1.05; P = 0.11). However, a significant heterogeneity was also found across these studies (I2 = 93%; P < 0.00001) ().
Publication bias
Funnel plot with standard errors was used to assess the publication bias among studies. The symmetric funnel plot implied that no publication bias was present in these pooled analyses (Fig. S4).
Subgroup analysis and Meta-regression
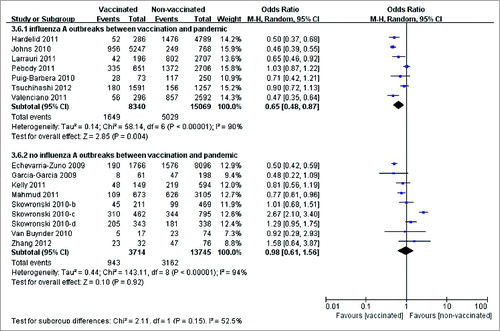
Subgroup analysis was performed to investigate the potential effect of related regional factors according to a pre-specified protocol. Grouping was based on the study location and the influenza activities in the period between seasonal vaccination and pandemic influenza circulation (Table S9). Subgroup analysis was only performed for case-control studies because of the limited number of RCTs and cohort studies. Two subgroups were derived from 16 case-control studies that provided data on laboratory-confirmed pandemic cases. Significant positive results observed in the subgroup that influenza A outbreaks were detected before the 2009 pandemic, for which the vaccine effectiveness was 35%. However, no cross-protection from seasonal influenza vaccine was detected based on the pooled effect of the other subgroups (OR = 0.98; 95% CI: 0.61, 1.56; P = 0.92). In addition, significant heterogeneity was observed across studies in both subgroups ().
Discussion
Our updated systematic review and meta-analyses suggested a moderate effect of the seasonal influenza vaccine on the reduction of laboratory-confirmed pandemic 2009 A (H1N1) influenza infection. The vaccine effectiveness was 20% in the national combined data (according to 16 case-control studiesCitation5-7,Citation9-10,Citation13-15,Citation25,27,Citation29-32), and 35% in several regions. However, the results also showed a potential association between the seasonal vaccine receipt and increase risk of pandemic influenza infection among certain groups of people (RR = 1.27 based on RCTsCitation11,18,19). In addition, no publication bias was detected in the meta-analysis, and the results indicated that seasonal influenza vaccine may confer some protection against ILIs. A significant 9% risk reduction was detected among RCTs. However, different results were observed among cohort and case-control studies, which may be caused by the differences in the study designs and potential effects from bias and confoundings.
Our findings suggested that seasonal influenza vaccine can provide protection against ILI. Our analysis supported the hypothesis that a modest cross-protection against laboratory-confirmed illness was found, which was in agreement with the observations from previous immunology studies.Citation36-39 A possible explanation for this cross-protection can be that seasonal influenza vaccine may boost cross-reactive antibodies, and therefore, confers non-specific protection against other influenza strains. Evidence from previous serological studies support this hypothesis. The results of serological analysis showed that seasonal influenza vaccine can increase the hemaglutination inhibition and neutralization titers against the 2009 pandemic A (H1N1) influenza virus.Citation36,37 Furthermore, studiesCitation38,39 have reported differences in the immunoreaction expressed at the level of T-cell epitopes, resulting from seasonal influenza vaccine. In addition, similar findings were observed for the previous 1918 and 1968 pandemic strains.Citation40,41
However, we must be note that some inconsistencies were also present among the meta-analyses for RCTs, and cohort and case-control studies. The signal of partial protection was mainly drawn from case-control studies, in which heterogeneity was high. Moreover, the meta-analyses of RCTs showed different results for several particular study settings. However, we have to note that aside from the study design, the RCTs included in the meta-analysis had several different characteristics from the other studies. The three RCTs were performed in Hong Kong, and the participants were school-age children who were recruited via schools. A higher-risk ratio of the pandemic influenza infection between seasonal vaccinated and control groups was reported in this RCT. In addition, the number of cases in RCTs was small. These findings from RCTs may be insufficient to generalize the general population because of the limitations in the study setting.
A number of potential explanations were found for these controversial results. The first explanation suggested that a selection bias or unidentified confounders were found in several of the studies that resulted in the unexpected findings. However, this explanation may not be true according to several investigations, which confirmed that an unidentified confounder is unlikely to cause the apparent risk increase of pandemic influenza infection and seasonal influenza vaccination.Citation42 Other explanations suggested the association between seasonal influenza vaccination and pandemic A (H1N1) infection resulted from several biological mechanisms. One of these mechanisms was based on the concept of “temporary immunity,” which refers to a broad non-specific immunity lasting for 3 to 6 months elicited by influenza virus infections. This mechanism confers a broad protection against infection compared with any other influenza virus when first elicited, but such protection wanes over a short time frame.Citation17 A possibility was found in which both seasonal influenza infection and seasonal influenza vaccination may cause induced similar “temporary immunity,” and therefore, provide a short-term cross-protection against the 2009 pandemic infection. Several studies have reported that antibodies binding to the stalk of the hemagglutinin of an influenza virus can provide broad heterosubtypic immunity.Citation43,44 This kind of antibodies can also be induced by vaccination, but will only last for a short period. This phenomenon may explain the varying results of the impacts on pandemic A (H1N1) infection from seasonal influenza vaccines. Based on this hypothesis, the local seasonal influenza activities in the period between seasonal vaccination and pandemic influenza circulation and the time of vaccination and participant recruitment may affect the findings. In addition, we must note the gap between vaccine efficacy or effectiveness and immunogenicity.Citation45 Inconsistencies may be present when comparing the results from these studies. Such inconsistencies may also give rise to controversy.
This updated meta-analysis differs from the previously published meta-analysisCitation20 in several ways. First, we included 6 more studies published up to October 2013 to estimate the pooled effect and draw a conclusion, which may help to provide a more updated and comprehensive result. Second, a subgroup analysis was performed to determine the potential effects of previous circulating influenza strains on the association between the receipt of seasonal influenza vaccination and the 2009 pandemic influenza infection. Moreover, the findings included the protective efficacy for a particular age group (children aged 6 to 17 years old).
Although this meta-analysis provides helpful information, several potential bias areas still exist at the study and outcome level, as well as at review-level. First, significant heterogeneity was detected in 2 out of 6 meta-analyses. For instance, high heterogeneity with I2 = 93% was detected in the pooled estimate of the seasonal influenza vaccine compared with the laboratory-confirmed pandemic infection across the case-control studies, even though the analysis utilized various settings. Hence, a random effect model was used. Moreover, a subgroup analysis based on the study location and previous circulating influenza strains was performed to explore the cause of the heterogeneity. Second, a test-negative case-control study design was used in several included studies at study level.Citation15,17,29,30 Citation47,48,49 Third, the results were unable to adjust for the age groups, which is significantly difficult in practice. Nineteen of the included studies enrolled participants from all age groups. In addition to the influenza strains and vaccine component, age may significantly influence the immune response. Moreover, especially for the 2009 pandemic, cross-reacting antibodies were detected in older people born in 1930 or earlier.Citation47-50 Although separately estimating the effect on the elderly is impossible, the findings included specific effects of seasonal influenza on school-age children in Hong Kong. Finally, similar with other meta-analyses, the raw data was used to calculate the pooled effect. Several adjustments for possible confounders were unavailable in this meta-analysis. For the studies by Hardelid et al.Citation9 and Valenciano et al.Citation10, although the crude data showed cross-protection from seasonal influenza vaccine, no association was found between vaccine and pandemic infection after adjustment for sex, age, and other potential confounders.
Methods
This systematic review and meta-analyses was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.Citation51
Eligibility criteria
We systematically searched for all RCTs, cohort studies, and case-control studies involving the assessment of vaccine efficacy or effectiveness against the 2009 pandemic A (H1N1) influenza of seasonal influenza vaccination published since 2009. The eligibility criteria were as follows: (1) Types of participants: We included studies focusing on seasonal influenza vaccines with no limits in the age, sex, health status, and geographical location; (2) Types of interventions: We included seasonal vaccinations in the northern hemisphere for the 2007–2008, 2008–2009, and /or 2009–2010 influenza seasons and seasonal vaccinations in the southern hemisphere for the 2008 and/or 2009 influenza seasons; (3) Types of controls: For RCTs and cohort studies, controls were defined as participants who did not receive seasonal influenza vaccination for the 2008 and/or 2009 influenza seasons. For case-control studies, controls were defined as laboratory-negative pandemic influenza A (H1N1) subjects; (4) Types of outcome measures: The primary outcome measure was laboratory-confirmed A (H1N1) influenza infection detected through serological method or RT-PCR. Moreover, the secondary outcomes include clinically defined ILI, acute respiratory illness, and absenteeism. We excluded studies if they reported: (1) animal studies; (2) in languages other than English and Chinese; (3) letters, reviews, and other types of articles without the original data; and (4) studies that only reported on the seasonal influenza vaccine for other seasons, those considered as low-quality studies, and those that had high risk of bias.
Literature search
Searches were performed using PubMed (January 1960 to October 2013), MEDLINE (January 1946 to October Week 1 2013), Ovid Embase Classic + Embase (1947 to 2013 Week 41), The Cochrane Library databases (Issue 7 of 12, October 2013), SCOPUS, and China National Knowledge Infrastructure for publications after 2009 and limited to humans regardless of language. In addition, the reference lists of the retrieved reviews were hand-searched to identify further studies of interest. For unpublished trials, the authors were also directly contacted to request for missing information.
A full list of search terms for all databases was provided. For instance, for PubMed, the search terms were "Influenza, Human"[Mesh] OR influenza OR flu AND "Influenza A virus, H1N1 subtype"[Mesh] OR swine OR porcine OR H1N1 AND "Influenza Vaccines"[Mesh] OR vaccines OR "influenza vaccines" OR "seasonal influenza vaccines" AND efficacy OR effectiveness OR effic$ OR effect$ AND “Pandemics”[Mesh] OR “Disease Outbreaks”[Mesh]. The databases were also limited to studies with human subjects, published since 2009, and without other dates or language limitations. Similar search terms with minor variations based on the database structure were used in other databases.
Study selection and data extraction
Endnote software (version X7, Thomson Reuters, New York, NY) was used to manage the citations and studies. Two reviewers independently screened the citations according to the eligibility criteria. Of the 1076 identified articles, 1027 were excluded after title and abstract screening. For the remaining 49 records that could not be clearly categorized according to the above procedure, their full texts were retrieved for close examination. Twenty studies were further excluded according to the inclusion and exclusion criteria.
Data extraction was independently performed and in duplicates using a standardized data extraction form by Li and Chen. Any disagreement was settled through discussion after carefully reviewing the articles. The following data on the baseline characteristics were extracted: lead author, year of publication, study location, study design, enrollment period, number of subjects for each arm, characteristics of participants (age, sex, and other relevant details of participants), details of intervention, and associated outcome events and funding.
Quality assessment
For RCTs, the Jadad scale was used to assess the quality of the included studies. The scale consists of 5 questions focusing on the reliability, validity, and responsiveness of a study.Citation52 With a maximum score of 5 for each trial, trials that scored 2 points or below were considered as low-quality studies and were excluded in the meta-analysis.Citation53 We also assessed the potential bias of each individual trail according to Cochrane Handbook of Systematic Reviews of Interventions, including the selection bias, performance bias, detection bias, attritionbias and reporting bias.Citation54
The Newcastle-Ottawa Scale (NOS) was chosen to assess the quality of the cohort and case-control studies.Citation55 NOS uses a “star system,” in which the following 3 aspects of a study are judged: the selection of the study group, the comparability of the groups, and the ascertainment of either the exposure or outcome. NOS contains 8 items, and a maximum of 9 stars can be allocated for a study. The risk of bias from the cohort or case-control studies was classified into the following 3 categories:
Low risk of bias: any study scoring ≥ 7 stars and at least has 1 star in each domain;
Moderate risk of bias: any study scoring between 4 to 6 stars;
High risk of bias: any study scoring ≤ 3 stars.
Statistical analysis
The analysis was performed using the software Review Manager 5.2 (Review Manager. Version 5.2. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration; 2012.) and R package (Version 3.0.2). Pooled effects were reported as RRs or ORs for the RCTs or cohort studies, and for case-control studies, respectively, using Mantel-Haenszel (M-H) method with a random effect model.Citation56 The corresponding 95% CIs and 2-sided P value were also presented. The cross-protection of the seasonal influenza vaccine is defined as 1- RR for the RCTs or cohort studies, and 1- OR for the case-control studies. ICitation2 statistics was used to assess the heterogeneity of each pooled estimate. In this meta-analysis, the categorization of the values for I2 was adopted from Higgins,Citation57,58 and low, moderate, and high adjectives were assigned to the I2 values of 25, 50, and 75%. The results (RR or OR) were regarded as statistically significant if P < 0.05 and the corresponding 95% CI did not include 1. The publication bias was graphically assessed with Begg's funnel plot.Citation59 For additional analyses, a pre-specified subgroup analysis was performed based on the study locations and previous circulating influenza viruses for laboratory-confirmed pandemic cases.
Conclusion
Seasonal influenza vaccines are crucial components for the control of influenza. This study provided increased assurance that receiving a 2009 seasonal influenza vaccine before a pandemic is not harmful and is even beneficial for adults. Further immunological studies are needed to understand the mechanism underlying these findings because of the limited number of included RCTs and cohort studies, as well as the high heterogeneity among the included case-control studies. Furthermore, future research may explore the differences in the temporary immunity response elicited by previous infections of heterosubtypic and seasonal influenza vaccinations, as well as their effects on pandemic influenza infection. In addition, studies aiming to obtain more effective and universal influenza vaccines, which can induce broad-spectrum immune response, are extremely important for the development of influenza vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
Supplemental_Material.docx
Download MS Word (71.4 KB)Acknowledgments
The authors are grateful to Dr. Ben Cowling in the University of Hong Kong for his special assistance.
References
- Sekkides O. Pandemic influenza—a timeline. Lancet Infect Dis 2010; 10:663; PMID:20931693
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, et al. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science 2009; 325:197-201PMID:19465683
- Malik Peiris J, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol 2009; 45(3):169-73; PMID:19540800; http://dx.doi.org/10.1016/j.jcv.2009.06.006
- World Health Organization. Global alert and response (GAR). [cited February 2014]; Available from: http://www.who.int/csr/don/2010_05_14/en/
- Echevarría-Zuno S, Mejía-Aranguré JM, Mar-Obeso AJ, Grajales-Muñiz C, Robles-Pérez E, González-León M, Ortega-Alvarez MC, Gonzalez-Bonilla C, Rascón-Pacheco RA, Borja-Aburto VH. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. The Lancet 2010; 374(9707):2072-9; PMID:19913290; http://dx.doi.org/10.1016/S0140-6736(09)61638-X
- Garcia-Garcia L, Valdespino-Gómez JL, Lazcano-Ponce E, Jimenez-Corona A, Higuera-Iglesias A, Cruz-Hervert P, Cano-Arellano B, Garcia-Anaya A, Ferreira-Guerrero E, Baez-Saldaña R, et al. Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: case-control study in Mexico City. BMJ (Clinical research ed) 2009; 339:b3928; PMID:19808768
- Johns MC, Eick AA, Blazes DL, Lee SE, Perdue CL, Lipnick R, et al. Seasonal influenza vaccine and protection against pandemic (H1N1) 2009-associated illness among US military personnel. PloS one. 2010; 5(5):e10722; PMID:20502705; http://dx.doi.org/10.1371/journal.pone.0010722
- Orellano PW, Reynoso JI, Carlino O, Uez O. Protection of trivalent inactivated influenza vaccine against hospitalizations among pandemic influenza A (H1N1) cases in Argentina. Vaccine. 2010 Jul 19; 28(32):5288-91; PMID:20541580; http://dx.doi.org/10.1016/j.vaccine.2010.05.051
- Hardelid P, Fleming DM, McMenamin J, Andrews N, Robertson C, Sebastian Pillai P, Ellis J, Carman W, Wreghitt T, Watson JM, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A (H1N1) 2009 infection in England and Scotland 2009–2010. Euro surveill 2011; 16(2); PMID:21251487
- Valenciano M, Kissling E, Cohen JM, Oroszi B, Barret AS, Rizzo C, Nunes B, Pitigoi D, Larrauri Cámara A, Mosnier A, et al. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) multicentre case-control study. PLoS Med. 2011; 8(1):e1000388; PMID:21379316
- Cowling BJ, Ng S, Ma ESK, Fang VJ, So HC, Wai W, Cheng CK, Wong JY, Chan KH, Ip DK, et al. Protective efficacy against pandemic influenza of seasonal influenza vaccination in children in Hong Kong: A randomized controlled trial. Clin Infect Dis 2012; 55(5):695-702; PMID:22670050; http://dx.doi.org/10.1093/cid/cis518
- Richmond P, et al. A Study of the Efficacy, Safety and Tolerability Profile of CSL Limited's Influenza Virus Vaccine (CSL's IVV) Administered Intramuscularly in Healthy Adults) ClinicalTrials.gov Identifier: NCT00562484. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT00562484?term=NCT00562484&rank=1
- Kelly HA, Grant KA, Fielding JE, Carville KS, Looker CO, Tran T, Jacoby P. Pandemic influenza H1N1 2009 infection in Victoria, Australia: No evidence for harm or benefit following receipt of seasonal influenza vaccine in 2009. Vaccine 2011 ; 29(37):6419-26; PMID:21473950; http://dx.doi.org/10.1016/j.vaccine.2011.03.055
- Van Buynder PG, Dhaliwal JK, Van Buynder JL, Couturier C, Minville-Leblanc M, Garceau R, et al. Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respi Viruses. 2010; 4(4):171-8; PMID:20629771; http://dx.doi.org/10.1111/j.1750-2659.2010.00146.x
- Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, Rosella LC, Dickinson JA, Gilca R, Sethi P, et al. Association between the 2008–2009 seasonal influenza vaccine and pandemic H1N1 illness during Spring to Summer 2009: Four observational studies from Canada. PLoS Med 2010; 7(4):e1000258; PMID:20386731
- Jefferies S, Earl D, Berry N, Blackmore T, Rooker S, Raymond N, Pritchard A, Weatherall M, Beasley R, Perrin K. Effectiveness of the 2009 seasonal influenza vaccine against pandemic influenza A (H1N1) 2009 in healthcare workers in New Zealand, June to August 2009. Euro Surveill. 2011; 16(2); PMID:21251486
- Kelly H, Barry S, Laurie K, Mercer G. Seasonal influenza vaccination and the risk of infection with pandemic influenza: a possible illustration of non-specific temporary immunity following infection. Challenge 2010; 16:17.
- Cowling BJ, Ng S, Ma ESK, Cheng CKY, Wai W, Fang VJ, Chan KH, Ip DK, Chiu SS, Peiris JS, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 2010; 51(12):1370-9 PMID:21067351
- Klick B, Durrani S, Chan KH, Ip DKM, Chou ESK, Kwok HKH, Ng S, Chiu SS, Peiris JS, Leung GM, et al. Live attenuated seasonal and pandemic influenza vaccine in school-aged children: A randomized controlled trial. Vaccine. 2013; 31(15):1937-43; PMID:23434387; http://dx.doi.org/10.1016/j.vaccine.2013.02.017
- Yin JK, Chow MYK, Khandaker G, King C, Richmond P, Heron L, Booy R. Impacts on influenza A (H1N1) pdm09 infection from cross-protection of seasonal trivalent influenza vaccines and A (H1N1) pdm09 vaccines: Systematic review and meta-analyses. Vaccine 2012; 30(21):3209-22; PMID:22387221; http://dx.doi.org/10.1016/j.vaccine.2012.02.048
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. Morbidity and Mortality Weekly Report 2009; 58(RR8):1–51
- Carcione D, Giele C, Goggin LS, Kwan KS, Smith DW, Dowse GK, Mak DB, Effler P. Association between 2009 seasonal influenza vaccine and influenza-like illness during the 2009 pandemic: preliminary results of a large household transmission study in Western Australia. Euro Surveill 2010; 15(28); PMID:20650055
- Yin JK, Wang H, Skinner SR, Salkeld G, Booy R. Assessing seasonal vaccine-related cross-protection from 2009 pandemic H1N1 influenza through teacher absenteeism. Aust N Z J Public Health 2011; 35(4):393-4; PMID:21806738; http://dx.doi.org/10.1111/j.1753-6405.2011.00748.x
- Janjua NZ, Skowronski DM, Hottes TS, Osei W, Adams E, Petric M, Sabaiduc S, Chan T, Mak A, Lem M, et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: first detection of the association in British Columbia, Canada. Clin Infect Dis 2010; 51(9):1017-27; PMID:20887210; http://dx.doi.org/10.1086/656586
- Larrauri A, Savulescu C, Jimenez-Jorge S, Perez-Brena P, Pozo F, Casas I, Ledesma J, de Mateo S; Spanish Influenza Surveillance System (SISS). Influenza pandemic (H1N1) 2009 activity during summer 2009. Effectiveness of the 2008 to 2009 trivalent vaccine against pandemic influenza in Spain. Gaceta Sanitaria 2011; 25(1):23-8; PMID:21334788
- Danielle Luliano AA, Reed C, Gun A, Desai M, Dee DL, Kutty P, Gould LH, Sotir M, Grant G, Lynch M, et al. Notes from the field: outbreak of 2009 pandemic influenza A (H1N1) virus at a large public University in Delaware, April to May 2009. Clin Infect Dis 2009; 49(12):1811-20; PMID:19911964; http://dx.doi.org/10.1086/649555
- Mahmud SM, Van Caeseele P, Hammond G, Kurbis C, Hilderman T, Elliott L. No association between 2008–09 influenza Vaccine and Influenza A (H1N1) pdm09 virus infection, Manitoba, Canada, 2009. Emer Infecti Dis 2012; 18(5):801; PMID:22516189; http://dx.doi.org/10.3201/eid1805.111596
- Nelson CA, France EK, Shetterly SM, Glanz JM. Seasonal influenza vaccination status among children with laboratory evidence of pandemic H1N1 infection. Pediat Infect Dis J 2011; 30(7):562-5; PMID:21248657; http://dx.doi.org/10.1097/INF.0b013e31820bb482
- Pebody R, Andrews N, Waight P, Malkani R, McCartney C, Ellis J, Miller E. No effect of 2008/09 seasonal influenza vaccination on the risk of pandemic H1N1 2009 influenza infection in England. Vaccine 2011; 29(14):2613-8; PMID:21292008; http://dx.doi.org/10.1016/j.vaccine.2011.01.046
- Puig-Barbera J, Arnedo-Pena A, Pardo-Serrano F, Tirado-Balaguer MD, Perez-Vilar S, Silvestre-Silvestre E, Calvo-Mas C, Safont-Adsuara L, Ruiz-García M; Surveillance and Vaccine Evaluation Group during the autumn 2009 H1N1 pandemic wave in Castellón, Spain. Effectiveness of seasonal 2008–2009, 2009–2010, and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellon, Spain. A test-negative, hospital-based, case-control study. Vaccine 2010; 28(47):7460-7PMID:20875486
- Tsuchihashi Y, Sunagawa T, Yahata Y, Takahashi H, Toyokawa T, Odaira F, Ohyama T, Taniguchi K, Okabe N. Association between seasonal influenza vaccination in 2008–2009 and pandemic influenza A (H1N1) 2009 infection among school students from Kobe, Japan, April to June 2009. Clin Infect Dis 2012; 54(3):381-3; PMID:22100572
- Xiang Z, Yi-lin H, Hai-yun Y. A retrospective evaluation of protective effect of seasonal influenza vaccine and influenza A (H1N1) vaccine. Chinese J Dis Cont Prevent ISTIC 2012; 16(5):452–54
- Gargiullo P, Shay D, Katz J, Bramley A, Nowell M, Michalove J, Kamimoto L, Singleton JA, Lu PJ, Balluz L, et al. Effectiveness of 2008–09 trivalent influenza vaccine against 2009 pandemic influenza A (H1N1) – United States, May–June 2009. Morbidity and Mortality Weekly Report 2009; 58(44):1241–5
- Lessler J, Reich NG, Cummings DA. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med. 2009; 361(27):2628-36; PMID:20042754; http://dx.doi.org/10.1056/NEJMoa0906089
- Pelat C, Falchi A, Carrat F, Mosnier A, Bonmarin I, Turbelin C, Vaux S, van der Werf S, Cohen JM, Lina B, et al. Field effectiveness of pandemic and 2009–2010 seasonal vaccines against 2009–2010 A (H1N1) influenza: estimations from surveillance data in France. PloS One. 2011; 6(5):e19621; PMID:21573005
- Corti D, Suguitan Jr AL, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest 2010; 120(5):1663; PMID:20389023; http://dx.doi.org/10.1172/JCI41902
- Bethell D, Saunders D, Jongkaewwattana A, Kramyu J, Thitithayanont A, Wiboon-ut S, Yongvanitchit K, Limsalakpetch A, Kum-Arb U, Uthaimongkol N, et al. Evaluation of in vitro cross-reactivity to avian H5N1 and pandemic H1N1 2009 influenza following prime boost regimens of seasonal influenza vaccination in healthy human subjects: a randomised trial. PloS One 2013; 8(3):e59674.
- De Groot AS, Ardito M, McClaine EM, Moise L, Martin WD. Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008–2009 conventional influenza vaccine. Vaccine 2009; 27(42):5740-7; PMID:19660593; http://dx.doi.org/10.1016/j.vaccine.2009.07.040
- Echevarría-Zuno S, Mejía-Aranguré JM, Grajales-Muñiz C, Gonzalez-Bonilla C, Borja-Aburto VH. Seasonal vaccine effectiveness against pandemic A/H1N1 - Authors' reply. The Lancet. 2010; 375(9717):802-3; http://dx.doi.org/10.1016/S0140-6736(10)60339-X
- Tumpey TM, García-Sastre A, Taubenberger JK, Palese P, Swayne DE, Basler CF. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc Natl Acad Sci U S A. 2004; 101(9):3166-71; PMID:14963236
- Mogabgab WJ, Leiderman E. Immunogenicity of 1967 polyvalent and 1968 Hong Kong influenza vaccines. JAMA. 1970; 211(10):1672-6; PMID:4984268
- Crowcroft NS, Rosella LC. The potential effect of temporary immunity as a result of bias associated with healthy users and social determinants on observations of influenza vaccine effectiveness; could unmeasured confounding explain observed links between seasonal influenza vaccine and pandemic H1N1 infection? BMC Public Health 2012; 12:458; PMID:22716096; http://dx.doi.org/10.1186/1471-2458-12-458
- Ekiert DC, Bhabha G, Elsliger M-A, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science 2009; 324(5924):246-51; PMID:19251591
- Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, et al. Haemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci 2012; 109(7):2573-8; PMID:22308500; http://dx.doi.org/10.1073/pnas.1200039109
- Gilbert PB, Qin L, Self SG. Evaluating a surrogate endpoint at three levels, with application to vaccine development. Stat Med 2008; 27(23):4758-78; PMID:17979212; http://dx.doi.org/10.1002/sim.3122
- Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, Orenstein WA. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol 2007; 36(3):623-31; PMID:17403908; http://dx.doi.org/10.1093/ije/dym021
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361(20):1945-52; PMID:19745214
- Booy R, Khandaker G, Heron LG, Yin J, Doyle B, Tudo KK, Hueston L, Gilbert GL, Macintyre CR, Dwyer DE. Cross-reacting antibodies against the pandemic (H1N1) 2009 influenza virus in older Australians. Med J Aust 2011; 194(1):19; PMID:21449863
- Ikonen N, Strengell M, Kinnunen L, Osterlund P, Pirhonen J, Broman M, et al. High frequency of cross-reacting antibodies against 2009 pandemic influenza A (H1N1) virus among the elderly in Finland. Euro Surveill 2010; 15(5):19478; PMID:20144443
- Katz J, Hancock K, Veguilla V, Zhong W, Lu XH, Sun H, Butler E, Dong L ,Liu F, Li ZN. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine (Reprinted from MMWR, vol 58, 2009, pp 521–524, 2009). Jama-Journal of the American Medical Association 2009; 302(3):249–50
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annal Int Med 2009; 151(4):264-9; PMID:19622511; http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00135
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Cont Clin Ttrial 1996; 17(1):1–12
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8(1):18
- Higgins J, Green S. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). [cited 11 February 2013]; Available from: www.cochranehandbook.org.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute URL: http://www.ohrica/programs/clinical_epidemiology/oxford_htm Accessed on. 2012; 2.
- Bailey K R. Inter-study differences: how should they influence the interpretation and analysis of results? Stat Med 1987; 6(3): 351-358.
- Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11):1539-58; PMID:12111919; http://dx.doi.org/10.1002/sim.1186
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414):557; PMID:12958120
- Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst 1989; 81(2):107-15; PMID:2642556; http://dx.doi.org/10.1093/jnci/81.2.107