Abstract
To improve lower efficacy among infants in low income countries and the safety (e.g., rare but severe intussusception) of live oral rotavirus vaccines, we have developed CDC-9 strain with G1P[8] specificity as a candidate inactivated rotavirus vaccine (IRV). This IRV of 3 doses elicits high titers of IgG, neutralizing activity to homotypic and heterotypic human strains and IgG avidity in guinea pigs, thus is a promising alternative to enhance global immunization against rotavirus in children.
To improve the safety (e.g.,, rare but severe intussusception) and lower immunogenicity and efficacy of live oral rotavirus vaccines among infants in low income countries, we have developed CDC-9 strain with G1P[8] specificity as a candidate inactivated rotavirus vaccine (IRV).Citation1,2 This strain possesses desired biophysical, biochemical and virological characteristics for an IRV, as it grows to high titer (up to 108 ffu/ml) in Vero cells and maintains predominant triple-layered particles (>95%) during upstream manufacture and downstream purification processes. Inactivated CDC-9 strain alone when administered to skin using a microneedle patch was highly immunogenic even at a fractional dose of an intramuscular dose in mice.Citation3 Inactivated CDC-9 strain when formulated with alum adjuvant and administered intramuscularly induced robust antibody response and protection from an oral challenge with a virulent homotypic human strain in gnotobiotic piglets.Citation4
We further conducted studies to address the heterotypic immunity and reported that a monovalent IRV induced broad cross-reactive neutralizing activity (NA) against human G1P[8], G9P[8], and G8P[4] strains in gnotobiotic piglets and guinea pigs.Citation5 Regrettably, we reported rotavirus-specific IgG and NA in sera of IRV-immunized guinea pigs on incorrect days of post vaccination. Here we present correct kinetic data on homotypic and heterotypic antibody response in immunized guinea pigs (new ). One dose of IRV significantly increased titers of IgG and NA against the 2 human strains, homotypic Wa G1P[8] (mean = 320, p = 0.005) and heterotypic MW333 G8P[4] (mean = 176, p = 0.005). Meanwhile, the NA against the bovine WC3-human WI79 reassortant (G1P[5]) was low (mean = 28) and undetectable against the parent WC3 bovine strain. A second dose boosted IgG and NA against the strains Wa (mean = 2048; p = 0.008) and MW333 (mean = 704; p = 0.010) as well as the G1P[5] reassortant (mean = 176; p = 0.007). However, it barely improved NA against the WC3 strain (mean = 44). A third dose further boosted IgG and NA against the strains Wa (mean = 3,072) and MW333 (mean = 1,152), but did not enhance NA against the WC3 strain and the G1P[5] reassortant virus. Of note, NA titers to the G1P[5] reassortant were significantly higher than the WC3 virus at post doses 2 (p = 0.010) and 3 (p = 0.007). No NA to the bovine-human WC3-WI79 (G6P[8]) reassortant was detected in any of pre and post sera.
Table 1. Rotavirus specific IgG and neutralizing activity in IRV-vaccinated guinea pigs
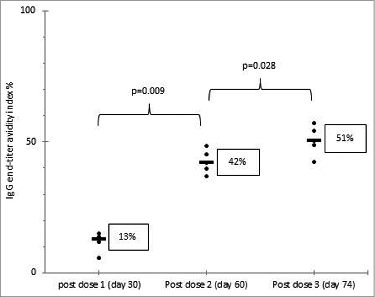
We also employed this collection of guinea pig sera to examine the kinetics and accumulated strength of IgG interactions with the multiple antigenic epitopes in virus particles by developing a rotavirus-specific IgG avidity assay (). All animals had undetectable levels of IgG or IgG avidity index in pre-bled sera. Like IgG titers, IgG avidity index increased to a median value of 13% after dose 1, 42% after dose 2 and 51% after dose 3. The increases in avidity index from dose 1 to dose 2 and from dose 2 to dose 3 were significant.
Figure 1. Shown are individual guinea pig IgG end-titer avidity index percentages (etAI%) and the medians of etAI% on post doses 1, 2 and 3. Statistical differences between the groups were determined by a Mann-Whitney U-test; the p-value ≤ 0.05 was considered significant. Rotavirus IgG in 5 guinea pig sera was measured by EIA and antibody titer was defined as the reciprocal of the highest dilution with a net OD value of greater than 0.1.Citation6 Rotavirus IgG avidity assay was performed by modifying the protocol of RV IgG EIA with the denaturant agent diethylamine (DEA). One dilution series started at 1:200 and was washed 6 times with 0.05% Tween 20-PBS, the other dilution series started at 1:20 and was washed 3 times for 5 min with 60 mM DEA in 0.05% Tween 20-PBS (pH 11.0) and 3 times with 0.05% Tween 20-PBS. etAI% were obtained using the formula etAI% = (end-titer DEA curve / end-titer wash buffer curve) X 100.Citation7
In summary, we have demonstrated that IgG and NA responses to IRV in guinea pigs were dose-dependent. While one dose of IRV was able to induce elevated IgG and NA against homotypic and heterotypic human strains, 2 doses were needed to prompt NA against the G1P[5] reassortant or the parent WC3 virus. A third injection further boosted the titers of IgG and NA to homotypic and heterotypic human strains but also augmented the avidity of IgG, thus a 3-dose regimen of a monovalent IRV is recommended for further clinical development in humans.
Disclosure of Potential Conflicts of Interest
Drs. Jiang and Glass and Ms. Wang hold patents through CDC for their work with inactivated rotavirus vaccine.
Acknowledgments
We thank W.J. Bellini and S. Mercader for assistance in developing rotavirus-specific IgG avidity assay and Stan Cryz of PATH for sponsoring the immunogenicity testing of IRV in guinea pigs. The finding and conclusions in this report are those of the authors and do not necessarily represent the official positions of Centers for Disease Control and Prevention.
References
- Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine 2008; 26:6754-8; PMID:18951937; http://dx.doi.org/10.1016/j.vaccine.2008.10.008
- Esona MD, Foytich K, Wang Y, Shin G, Wei G, Gentsch JR, Glass RI, Jiang B. Molecular characterization of human rotavirus vaccine strain CDC-9 during sequential passages in vero cells. Human Vaccin 2010; 6:247-253; PMID:20009519; http://dx.doi.org/10.4161/hv.6.3.10409
- Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine 2013; 31:3396-402; PMID:23174199; http://dx.doi.org/10.1016/j.vaccine.2012.11.027
- Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine 2010; 28:5432-6; PMID:20558244; http://dx.doi.org/10.1016/j.vaccine.2010.06.006
- Jiang B, Wang Y, Glass RI. Does a monovalent inactivated human rotavirus vaccine induce heterotypic immunity? evidence from animal studies. Human Vaccin Immunother 2013; 9:1634-7; PMID:23744507; http://dx.doi.org/10.4161/hv.24958
- Jiang B, Wang Y, Saluzzo JF, Bargeron K, Frachette MJ, Glass RI. Immunogenicity of a thermally inactivated rotavirus vaccine in mice. Human Vaccin 2008; 4:143-7; PMID:18382129; http://dx.doi.org/10.4161/hv.4.2.5263
- Mercader S, Garcia P, Bellini WJ. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings. Clinical Vaccin Immunol 2012; 19:1810-7; PMID:22971778; http://dx.doi.org/10.1128/CVI.00406-12
