Abstract
The nucleoprotein (NP) of influenza viruses is highly conserved and therefore has become one of the major targets of current universal influenza vaccine (UIV) studies. In this study, the recombinant nucleoprotein (NP) of the A/PR/8/34 (H1N1) influenza virus strain was expressed using an Escherichia coli (E. coli) expression system and then purified as a candidate UIV. The NP protein was administered intranasally or intraperitoneally twice at 3-week intervals to female BALB/c mice in combination with C48/80 adjuvant. Then, the mice were challenged with homologous or heterologous influenza viruses at a lethal dose 3 weeks after the last immunization. The results showed that the serum IgG titers of all of the mice immunized with NP reached a higher level and the protection provided by NP vaccine against the homologous virus depended on the administered dosage and adjuvant. In addition, immunization with 100 μg NP in combination with C48/80 adjuvant could provide good cross-protection against heterologous H9N2 avian influenza viruses. This study indicated that NP as a candidate antigen of UIV immunized intranasally could effectively induce mucosal and cell-mediated immunity, with the potential to control epidemics caused by the appearance of new emerging influenza viruses.
Introduction
Influenza is an acute respiratory disease caused by infection of the host respiratory tract by influenza virus, and vaccination is one of the most effective means against influenza virus infections. Currently, 2 surface glycoproteins, hemaglutinin (HA) and neuraminidase (NA), are the major antigen of influenza vaccines. However, HA and NA are prone to antigenic drift, and it is difficult to ensure antigens of vaccine strains match those in the strains circulating in the next season. Therefore, developing an effective and long-lasting universal influenza vaccine (UIV) has become a research priority at present. Once a pandemic influenza virus breaks out, it is a simply and rapid strategy to express and purify the antigen protein based on the expression system of bacteria, yeasts or baculoviruses.
The NP protein of influenza viruses is highly conserved and therefore has become one of the major targets of current UIV studies. Influenza virus nucleoprotein (NP) is type-specific, and is one of the main determinants of species specificity.Citation1 It has highly conserved sequences and low mutation rate during the evolution of viruses. NP proteins of influenza viruses of the same type have over 90% amino acid homology.Citation2 NP participates in the transcription and duplication of virus genome, and affects the host specificity and virulence of viruses.Citation3 Following infection of influenza virus, NP is the main antigen recognized by host cytotoxic T lymphocytes (CTL).Citation2 Through recognizing NP antigen peptides presented by MHC-I molecules on the surface of virus infected cells, CTL then kills virus infected cells and thereby eliminate the virus.Citation4 Cross-reactive CTL aimed at NP protein play an important role in controlling influenza virus infection.Citation2,4 There are many successful reports in the animal model studies about NP-based influenza vaccines. Citation5-8 Diverse vaccines with NP as the target antigen have been designed accordingly, including the diversely-designed DNA vaccines, the genetically engineered vaccines of insect baculovirus system expression and prokaryotic expression, as well as the viral vector vaccines,Citation5,6 which can induce certain heterosubtypic immune response. Moreover, recombinant protein vaccines have excellent safety, and can induce adequate antibody levels in a population vaccination, and therefore have become an important direction of influenza vaccine development at present.Citation9
However, if a new pandemic influenza virus breaks out, vaccination with a recombinant protein vaccine seems to be insufficient. Therefore, to further improve effectiveness of UIV, researchers tried to achieve the desired goals through combined use of multiple target antigens. In addition, recombinant protein vaccines require adjuvants to improve their ability of eliciting an effective immune response. Using a safe adjuvant with a vaccine will increase immune response and can lower dose of antigen required. Some adjuvants have been found to have the potential to enhance the immunogenicity of UIVs. For example, some mucosal adjuvants such as chitosan, lipid and E. coli thermosensitive toxin (LT) can induce balanced Th1/Th2 immune response by intranasal (i.n.) immunization in combination with vaccines.Citation10 Compound 48/80 (C48/80) is a polymer produced by the mixture of dimer, trimer and tetramer of concentrate of p-methoxyphenethyl-methylamine and formaldehyde, and plays a role of activating the mast cell (MC), so it is named MC activator.Citation11 C48/80 has been recognized and understood as a MC activator for a long time, but in recent studies, it is found to be a potential mucosal adjuvant. Just like other mucosal adjuvants, C48/80 can also induce the effective sIgA at the mucosa. In addition, C48/80 has also been confirmed as a nontoxic nasal adjuvant.Citation12
In this study, the NP protein was expressed using E. coli expression system and then purified as a subunit vaccine, and was immunized intranasally to mice in combination with C48/80 adjuvant. It was found that NP, as a candidate vaccine, could protect mice against the influenza virus challenge, and that C48/80 adjuvant could significantly enhance the protective effect of the NP vaccine.
Results
Intranasal administration of NP protein with C48/80 protected mice from lethal H1N1 virus challenge
To investigate whether intranasal administration of NP protein could protect mice against influenza virus infection, the Plasmid pET28a/NP was transformed into Escherichia coli (E.coli) BL21 (DE3) bacteria, and then expressed and purified the NP protein as a subunit vaccine as described by our previous study,Citation5 followed by immunized intranasally to mice with C48/80 adjuvant. 143 mice were divided randomly into 11 groups of 13 each (Group A–K, and S1). The mice (Groups A-H) were immunized by i.n. administration, and the mice (Groups I and J) were immunized intraperitoneally (i.p.) with 100 μg NP in combination with or without 60 μg C48/80 adjuvant. Group K was the unimmunized control. The mice intranasally immunized with 100, 30, 10 μg NP alone (Group B, D and F), or in combination with 60 μg C48/80 adjuvant (Group A, C and E). In addition, the mice intranasally immunized with C48/80 alone (Group G) were the adjuvant control, the mice intranasally immunized with 100 μg NP plus 1 μg CTB★ (Group H) were the positive control. Each mouse was i.n. or i.p. administered twice at an interval of 3 weeks with different amounts of NP with or without adjuvants. Three weeks after the last immunization, the mice were challenged intranasally with 10LD50 A/PR/8/34 (H1N1) virus suspension, and bronchoalveolar washes of 3 mice in each group were used for the analyses of virus loads in the lungs 3 d postinfection, while the rest of them were monitored to observe the survival rates and weight loss of the mice.
Table 1. Protection against lethal homologous influenza A virus challenge in mice by administration of NP vaccine with or without C48/80 adjuvant
The results showed that 100 μg NP protein with C48/80 adjuvant could enhance the immune effect after immunized mice. As shown in , 0% (0/10), 0% (0/10) and 40% (4/10) survival rates were obtained when challenges after immunized with 10 μg, 30 μg, and 100 μg NP protein alone (Group F, D and B), if the mice were immunizied with the same dosage of NP plus C48/80 adjuvant, higher levels of protection were achieved [20% (2/10), 40% (4/10) and 100% (10/10)] ( Group E, C and A). The mice injected intraperitoneally with 100 μg NP with adjuvant could not be effectively protected (, ). In , after intranasal immunization, the survival rate of mice in Group A, B, C and H had significant difference compared with unimmunized group (P < 0.05). And the survival rate of mice in Group A, C and H also had significant difference compared with the same doses of group without adjuvant (P < 0.05). As shown in , the group immunized with NP alone had a stronger protective effect along with the increased NP dose, and at the same time, the combination with C48/80 adjuvant could effectively enhanced protective immunity induced by NP.
Figure 1. Protection of mice against lethal challenge with homologous virus. Eleven groups of mice were immunized intranasally or intraperitoneal with various doses of NP vaccine alone or in combination with C48/80 adjuvant. The C48/80 immunized group, CTB★ immunized group and the unimmunized group served as adjuvant control, positive control, and negative control, respectively. Three weeks after the last immunization, mice were challenged with a lethal dose (10 × LD50) of influenza PR8 virus. Survival (A) and weight loss (B and C) were monitored for 21 d.
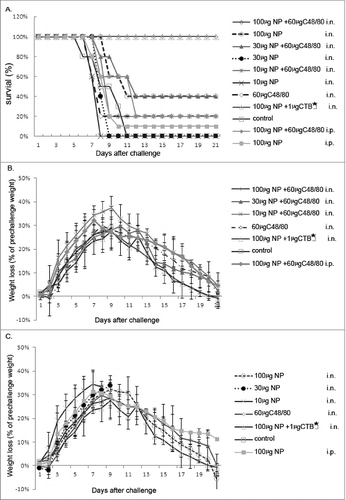
After influenza virus challenge, the results of body weight lost were consistent with survival rates in mice. As shown in the , the body weight loss was slightly slower and the symptoms was mildest in the mice immunized with 100 μg NP plus 60 μg C48/80 and 1 μg CTB★ adjuvant respectively (). The results also showed that the mice in Group B and Group D lost body weight slower, and they quickly recovered to the normal weight, while the rest of them in other groups began to recover their body weights 9 d after challenge.
As shown in , 3 d after challenge, the viral loads in the trachea/lung tissues from the mice that were immunized intranasally with 100 μg NP plus 60 μg C48/80 and 1 μg CTB★ adjuvant were significantly lower than that from the unimmunized control group (P < 0.05). In the i.n. immunized groups, the lung viral titers declined with increased NP doses regardless of the absence or presence of adjuvant, while the viral loads from the mice immunized with NP protein plus adjuvant were lower than that from the mice immunized with the same dose of NP alone. These results suggest that NP has the ability to provide protection for mice against influenza virus challenge, and just like CTB★, C48/80 adjuvant can significantly enhance the protective effect of the NP vaccine.
Antibody responses induced in mice immunized with NP protein
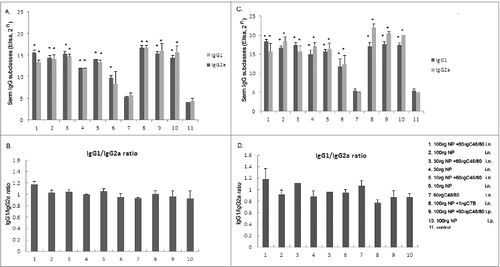
Mice in Group A-I were immunized as described above. Two weeks after the first and last immunization, the sera were obtained via caudal veins of mice in each group, followed by centrifugation for detection of IgG, IgG1 and IgG2a by ELISA. Three days after the challenge, the nasal washes were obtained from 3 mice in each group for detection of IgA by ELISA.
As shown in , after the first immunization, except the unimmunized control and adjuvant control group, the antibody titers induced in mice immunized intranasally or intraperitoneally were increased to varying extents. The group immunized with NP alone had higher antibody titer levels along with the increased NP dose; the group that was immunized with adjuvant had higher IgG antibody titer level compared to the group that was immunized with NP alone at a similar dose, with little difference between them. After the second immunization, except the unimmunized control group and adjuvant control group, each group had significantly higher IgG antibody titer level than that after the first immunization, but with little difference between them. Visibly, the IgG antibody titer level increased with the immunization times and the dosage of immunoprotein. Although the IgG level of the mice immunized intraperitoneally (Groups K and I) was comparable to that of the mice immunized mucosally with a corresponding dose, the mice could not be protected against virus infection (as shown in and ), indicating that the high titer of IgG Ab in the sera might not be the main cause to provide protection for mice against influenza virus infection.
Table 2. Antibody responses in mice induced by intranasal or intraperitoneal administration of NP vaccine with or without C48/80 adjuvant
At the same time, the nasal washes of intranasally immunized mice were obtained for detection of IgA. The groups immunized with 100 μg, 30 μg and 10 μg NP with adjuvant had higher IgA titers than the groups without adjuvant. The IgA titer increased with the immunized dosage of NP. Except the CTB★ control group, the group immunized with 100 μg NP plus C48/80 adjuvant had the highest IgA titer. IgA antibody was undetected in group G, I and J (). The aboved results indicated that the mice immunized with NP intranasally was able to effectively induce not only systemic immunity, but also mucosal immunity, producing a high level of mucosal IgA. The level of mucosal IgA might be correlated with the potential against the viral challenge.
Generally, IgG1 corresponds to Th2-biased responses, while IgG2a corresponds to Th1-biased responses. As shown in , the results showed that except the C48/80 adjuvant control group and the unimmunized group, the mice immunized with NP intranasally or intraperitoneally had high level of IgG1 and IgG2a, with little difference between them. However, the ratio of IgG1/IgG2a of the mice immunized with NP plus C48/80 was higher than that of the mice immunized with the same dosage of NP alone, regardless of the ratio after the first immunization or after the second immunization (). Regardless the first or the second immunization, except the group G, the IgG1 antibody titers in other groups all had significant differences compared with the control group (P < 0.05). After the first immunization, except the group F and G, the IgG2a antibody titers in other groups all had significant differences compared with the control group (P < 0.05), and after the second immunization, except the group G, the IgG2a antibody titers in other groups all had significant differences compared with the control group (P < 0.05).Therefore, it was speculated that with C48/80 adjuvant, the mice immunized with NP might induce quite balanced Th1/Th2 immune responses.
Figure 2. Antibody responses in mice induced by intranasal or intraperitoneal administration of NP vaccine with or without C48/80. Eleven groups of mice were immunized intranasally or intraperitoneal with various doses of NP vaccine alone or in combination with C48/80 adjuvant. The C48/80 immunized group, CTB★immunized group and the unimmunized group served as adjuvant control, positive control, and negative control, respectively. Two weeks after each immunization, serum of 3 mice in each group were prepared and examined by ELISA for NP-specific IgG1 and IgG2a Abs respectively. (A) The antibody responses of IgG1 and IgG2a detected in sera after the first immunization; (B) The ratio of IgG1/ IgG2a after the first immunization; (C) The antibody responses of IgG1 and IgG2a detected in sera after the second immunization; (D) The ratio of IgG1/ IgG2a after the second immunization. Results are expressed as mean ± SD of 3 tested mice in each group. *Displays Significant Difference with control group (P < 0.05).
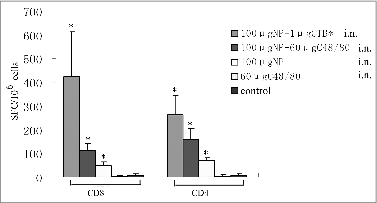
Cell-mediated immunity
In our previous study, the immunization with NP plus CTB★ adjuvant played its role not by neutralizing antibody, but through cell-mediated immunity.Citation5 To further define whether immunization with NP in combination with C48/80 can induce cellular immune responses, an ELISPOT assay was conducted. Influenza NP protein was highly conserved, especially many of its T-cell epitopes that have been shown to be completely conserved or individual amino acids differ by changes, but could still be recognized by the same T cells.Citation13 Therefore, we used 4 peptides recognized by CD8+ T cells and CD4+ T cells, respectively, as stimuli to detect the IFN-γ-secreting T cells by ELISPOT. As shown in , mice immunized intranasally with 100 μg NP plus C48/80 adjuvant induced more IFN-γ-secreting CD8+ T cells and CD4+ T cells, and the number of secreting CD4+ T cells was greater than that of secreting CD8+ T cells. Mice immunized intranasally with 100 μg NP plus C48/80 adjuvant induced more IFN-γ-secreting CD8+ T cells and CD4+ T cells than the mice immunized with NP alone at the corresponding dose, and there was significant difference compared to the mice immunized with C48/80 alone and the unimmunized mice (P < 0.05). In addition, the results also showed that the control mice immunized intranasally with 100 μg NP plus CTB★ adjuvant induced more IFN-γ-secreting CD8+ T cells and CD4+ T cells than the mice immunized with NP alone at the corresponding dose. These were consistent with our previous study results.
Figure 3. Detection of IFN-γ secreted by CD4+ or CD8+ (T) cells by Elispot. Mice were immunized intranasally with 100 μg NP vaccine alone or in combination with C48/80. The C48/80 immunized group, CTB★ immunized group and the unimmunized group served as adjuvant control, positive control, and negative control, respectively. Two weeks after the last immunization, the splenocytes were harvested and stimulated with peptides recognized by CD4+ T cell or CD8+ T cell. *Displays Significant Difference with control group (P < 0.05).
After spleen cells were stimulated by NP-related peptide as described above and cultured for 36 h, the content of NP-specific IFN-γ in the cell culture supernatant was analyzed by ELISA, and the results was also consistent with that in the ELISPOT experiment as described above (). The results showed that whether using MHC Class I peptide or MHC Class II peptide as stimuli, the mice immunized intranasally with 100 μg NP in combination with C48/80 adjuvant induced more IFN-γ than the mice immunized with NP alone at the corresponding dose, and there was significant difference compared to the mice immunized with C48/80 control or the unimmunized mice (P < 0 .05).
Table 3. Detection of IFN-γ secreted by CD4+ or CD8+ T cells from splenocytes after stimulation by ELISA
To sum up, NP protein can effectively induce cell-mediated immunity in mice immunized by mucosal routes, and induce NP-specific IFN-γ-secreting T cells. In addition, more IFN-γ-secreting T cells could be induced by using adjuvant.
Intranasal administration protected mice immunized with NP protein plus C48/80 against lethal challenge with homologous or heterologous influenza A virus
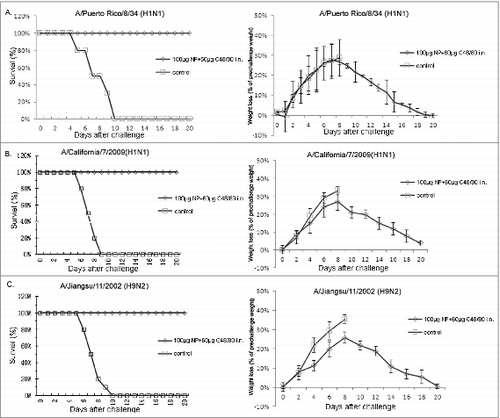
To explore whether the NP vaccine could provide protection against homologous and heterologous influenza viruses infection, seventy-eight BALB/c female mice (aged 6–8 weeks) were randomly divided into 2 groups. One group of mice were immunized with 100 μg NP plus C48/80 adjuvant as described above, and the anthor group of mice were the unimmunized control. Three weeks after the last immunization, all of the mice were challenged (i.n.) with 10 × LD50 A/ Chichen/Jiangsu/11/ 2002 (H9N2), A/Puerto Rico/8/34 (H1N1) and A/California/7/2009 NYMC X-179A (H1N1) viral suspension, respectively. As shown in and , compared to the unimmunized control mice, 100% (10/10) protection was obtained in the immunized group. Three days after challenge, the viral loads in the trachea/lung tissues from immunized mice were lower than that from the control mice (P < 0.05). The above results indicate that the internal protein NP with highly conserved sequences is able to provide protection against homologous virus infection, as well as providing cross-protection against heterosubtypic virus. Therefore, influenza virus NP could be an important candidate component for universal influenza vaccine.
Table 4. Protection against lethal homologous or heterologous influenza A virus challenge in mice by intranasal administration of NP vaccine with C48/80 adjuvant
Figure 4. Protection against lethal homologous or heterologous influenza A virus challenge in mice by intranasal administration of NP vaccine with C48/80 adjuvant. Mice were immunized intranasally with 100 μg NP vaccine in combination with 60 μg C48/80 adjuvant. Three weeks after the second immunization, mice were challenged with a lethal dose (10 × LD50) of A/Puerto Rico/8/34 (H1N1) (A), A/California/7/2009(H1N1) (B) and A/Jiangsu/11/2002 (H9N2) (C) influenza virus. Survival and weight loss were monitored for 21 d.
Discussion
The internal protein NP of influenza A virus is the major antigen as a candidate component of universal influenza vaccine due to its high conservative property. There are many successful reports in the animal models about the NP-based UIVs, and many studies have shown that the NP-based DNA vaccine and the virus vector vaccine not only can provide protection against homologous influenza virus, but also can resist to heterologous influenza virus challenge to some extent. It is generally considered that NP antibody could not effective neutralize virus, and the protective immunity is mainly mediated by NP-specific CTL immune response.Citation5 In 1987, Wraith D C et al. showed that immunization of mice intraperitoneal with NP purified from influenza virus X31 (H3N2) cultured in chick embryo cells would induce cross-reactive cytotoxic T cells, and this CTL reaction could protect 75% of mice against the lethal challenge of heterosubtypic influenza virus A/PR/8/34 (H1N1).Citation14
In this study, the NP vaccine with C48/80 adjuvant was able to provide the effectively protection for mice, which could resist to the lethal homologous and heterosubtypic influenza virus challenge. It is speculated that the immunization with NP protein in combination with C48/80 adjuvant can strengthen the CTL-mediated immune response, so as to clear the influenza virus infection, and that the type of cell-mediated immunity induced by C48/80 and CTB★ might be different to some extent. The C48/80-adjuvanted mice might induce the basis of quite balanced Th1/Th2 immune responsess.
However, recent studies suggested that antibodies against NP protein of influenza virus might have antiviral activity. LaMere et al.Citation15 injected NP-immune serum into naïve C57BL/6 mice once a day for 5 days, beginning 3 d before challenge infection. They found that the NP-specific IgG antibody titer significantly increased after several injections, and the virus load in lung of the mice injected with NP-immune serum were lower than that from the corresponding unimmunized mice. Therefore, in their opinion, a high titer of Ab is needed to engage antiviral mechanisms early postinfection. In our studies, the serological and cellular immunological experiments indicated that NP vaccine plus C48/80 adjuvant immunized by mucosal routes can effectively induce cell-mediated immunity and NP-specific IFN-γ-secreting T cells; in addition, the adjuvant can induce more IFN-γ-secreting T cells, thus protecting mice against influenza virus infection. Although we can't completely rule out the antibody role of NP which could not block the entry of influenza virus, CTL immune response induced by NP may play a critical role for clearing the influenza virus. Thus 3 d after influenza virus infection, we could see that the lung virus titers between NP plus adjuvant group and non-adjuvant group did not had significant difference, while the survival rate had significant difference.
Meanwhile, our study showed that mucosal immunity could also induce a high level of IgA Ab. The results indicated that mucosal immunization of mice with NP plus C48/80 adjuvant can effectively induce systemic immunity, as well as mucosal immunity that induced a high level of mucosal IgA. The level of mucosal IgA might be correlated with the potential against the viral challenge.Citation16,17 Tamura et al. confirmed that mice immunized intranasally with rNP from A/PR/8/34 expressed in insect cells could accelerate the clearing of the homologous virus in the nasal site of the mice, promoting recovery from infection.Citation18 Our study also confirmed that the protection immunity in mice was associated with the level of anti-NP IgA. It is possible that secretory IgA can react with the newly synthesized protein in the infected epithelial cells, thus affecting the replication and assembly of the virus, promoting recovery from infection.Citation5,19-21 These results indicated that NP as a candidate antigen of UIV immunized intranasally can effectively induce mucosal and cell-mediated immunity to provide protection against new emerging influenza viruses infection. Therefore, mucosal immunization might be more effective than other parenteral routes of immunization for clearing the virus, which might be due to the fact that recruitment of immune cells to the infection site in mice immunized intranasally occurred more quickly than in mice immunized parenterally.
In addition, C48/80 was an effective mucosal adjuvant. C48/80 was firstly discovered by Baltzly et al. in 1949,Citation22 who discovered that low dosage of C48/80 could reduce the blood pressure of mice when analyzing the hypotensive effect of isoquinoline derivatives. Subsequently, C48/80 has been recognized and understood as a MC activator for a long time, and studies have shown that the co-administration of MC activator micromolecule and antigen can significantly enhance the specific antibody responses of mice. MC activator might be an effective vaccine adjuvant. It is speculated that the combined use of MC activator and immunogenic protein or polypeptide might induce protective antigen-specific immune response.Citation23-25 Some studies showed that mast cells might promote the adaptive immunity through the effector function or by releasing various cytokines, chemokines and growth factors.Citation11 Therefore, recently, people have started to study the efficacy of MC activator C48/80 as an adjuvant, and C48/80 has been shown to be a potential mucosal adjuvant. Just like other mucosal adjuvants, C48/80 as a nontoxic nasal adjuvantCitation12 can also induce effective sIgA at the mucosal location, and clear the virus in the early stage of pathogen invasion, playing an immunological effect. However, up to now, the mechanism of action of C48/80 adjuvant has not been fully explored, and the related reports on C48/80 as an adjuvant in influenza vaccine were also very few. Xu et al. demonstrated that intranasal immunization of inactivated H1N1 virus with C48/80 elicited protective immunity against lethal challenge with homologous virus, but, could not provide protection when the immunogen was replaced with inactivated H5N1 virus.Citation26 Meng et al. also showed that the immunization of mice with C48/80 plus influenza HA expressed in eukaryotic cells could induce the balanced Th1/Th2 responses, preferentially inducing Th2-biased immune responses.Citation11 These were consistent with our experimental results. However, different from NP, HA as surface glycoprotein is prone to variation, and is not suitable to be used as the antigen of UIV. Our study showed that the mice immunized with 100 μg NP plus C48/80 adjuvant not only could resist to the homologous virus challenge well, but also could provide well cross-protection against heterologous H9N2 avian influenza virus infection. Therefore, NP as a candidate antigen of UIV immunized intranasally can effectively induce mucosal and cell-mediated immunity to provide protection against new emerging influenza virus infection.
In the study, the expressed and purified NP as a subunit vaccine was immunized intranasally to mice with C48/80 adjuvant. It was found that NP, as a candidate vaccine, could protect mice against the influenza virus infection, and that C48/80 adjuvant could enhance the protective effect of the NP vaccine. Recombinant NP protein was highly conserved and had definite ingredients, with no need for switching vaccine strains; therefore, this could be a way out for the issue of frequent vaccine strains switching in current seasonal flu vaccines. Under the circumstances when matching vaccine strains are not available, this method could effectively control an influenza epidemic of a new virus strain. However, our study of NP vaccine is intended as a proof-of-concept study, not as a final choice of vaccine. In addition, the duration of protection is important in vaccine development, actually, works for the development of long-term protective immunity of NP with C48/80 adjuvant are currently in progress.
Materials and methods
Viruses, mice and adjuvants
Mouse-adapted A/Puerto Rico/8/34 (H1N1), A/California/7/2009 NYMC X-179A (H1N1) and A/Chicken/Jiangsu/7/2002 (H9N2) influenza viruses were used in this study as described in our previous studies.Citation5,21,27,28 The H9N2 influenza virus was isolated by our group. The influenza virus NYMC X-179A was prepared by traditional reassortment methods possessing the HA, NA and PB1 genes of A/California/7/2009 (H1N1) virus, and PA, PB2, NP, M and NS genes of A/PR/8/34 virus. After being passaged and adapted in mouse, the viruses were aliquoted and stored at −70°C until use. All experiments with live H1N1 and H9N2 viruses were performed in a biosafety level 2 containment facility in SIBP (Shanghai Institute of Biological Products).
6 to 8 weeks old female BALB/c mice (specific-pathogen-free), were purchased from Shanghai Laboratory Animal Center, China. All mice were bred in the Animal Resource Center at Shanghai Institute of Biological Products and maintained in specific-pathogen-free conditions. All experiments involving animals have been approved by Animal Care Committee of Shanghai Institute of Biological Products.
Adjuvant C48/80, CT and CTB were purchased from Sigma (USA). One microgram of CTB supplemented with 2 ng of CT (0.2% of CTB), designated CTB★, was used as adjuvant.Citation5,18
Preparation of NP
Plasmid pET28a/NP was constructed by our lab in our previous study,Citation5 and then, the plasmid was transformed into Escherichia coli (E.coli) BL21 (DE3) bacteria for expression. They were add to LB medium supplemented containing 20 μg/ ml kanamycin, and incubated at 37°C until the culture reached OD600 of 0.6–0.8. Followed with 0.1 mM Isopropyl-β-d-thiogalactopyranoside (IPTG) in final concentration for inducing 8 h at 25°C, cells were harvested by centrifugation at 5,000 rpm for 5 min at 4°C. The target NP proteins were purified by Ni-NTA His-Bind Superfolw (Qiagen) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting as described in our previous study.Citation5 Protein concentrations in extracts were determined by the method of Bradford,Citation29 using bovine serum albumin (BSA) as the protein standard.
Immunization and challenge
Female BALB/c mice were anesthetized, followed by immunized intranasally or intraperitoneally 2 times with 3 weeks interval with PBS containing different doses of NP alone or in combination with 60 μg C48/80. The C48/80 adjuvanted-group taken as adjuvant control, the NP in combination with 1 μg CTB★ as passive control and the unimmunized group served as negative control. Mice were anesthetized and challenged intranasally with 20 μl of the viral suspension containing 10 × LD50 of A/Puerto Rico/8/34 (H1N1), 10 × LD50 of A/California/7/2009 (H1N1) and 10 × LD50 of A/Chicken/Jiangsu/7/2002 (H9N2) respectively, 3 weeks after the last immunization. Survival and weight loss were observed for 21 d.
Specimens
Two weeks after the first and the second immunization, serum of 3 mice in each group were randomly collected from the blood and used for NP-specific IgG, IgG2a and IgG1 Ab assays, respectively. Three days after the challenge, 3 mice from each group were randomly taken out for sample collection as described in our previous study.Citation5 The trachea and lungs were taken out and washed 3 times by injecting with a total of 2 ml of PBS containing 0.1% BSA, and the bronchoalveolar wash was centrifuged to remove cellular debris and used for virus titration. The head of the mouse was removed and the lower jaw was cut off. A syringe needle was inserted into the posterior opening of the nasopharynx and then a total of 1 ml of PBS containing 0.1% BSA was injected 3 times to collect the outflow as nasal wash. The nasal wash was used for IgA Ab assays after removing cellular debris.
Antibody (Ab) assays
The concentrations of IgG, IgG2a, IgG1 and IgA Abs against the NP were measured by ELISA. ELISA was performed using a series of reagents as described in our previous studies.Citation5,20,21 Goat anti-mouse IgG Ab (γ-chain specific) (KPL), goat anti-mouse IgG1 Ab(KPL), goat anti-mouse IgG2a Ab(KPL), and goat anti-mouse IgA (α-chain specific) (KPL) conjugated with horseradish peroxidase (HRP). The optical density was read at 450 nm. Ab-positive cut-off values were set as means + 2 × SD of unimmunized sera. An ELISA Ab titer was expressed as the highest serum dilution giving a positive reaction.Citation16,30
Measurement of cytokine release by stimulated splenocytes in mice
Spleen cells were isolated from mice for IFN-γ ELISPOT assays at 2 weeks after the last immunization, as described in our previous study.Citation5,7 The H-2d-restricted NP class I peptide (TYQRTRALV) and a pool of 3 H-2d-restricted class II peptides (FWRGENGRKTRSAYERMCNILKGK, RLIQNSLTIERMVLSAFDERNK, and AVKGVGTMVMELIRMIKRGINDRN) were used as stimulants.Citation3,31,32 The cell suspensions were stimulated in the presence of 10 μg/ml NP peptide stimulants. Spots were counted with an ELISpot reader system (Bioreader 4000; Bio-sys, Germany). The number of peptide-reactive cells was represented as spot forming cells (SFCs) per 106 splenocytes and was calculated by subtracting spot numbers in medium-only wells from spot numbers in peptide-containing wells.
Isolated spleen cells from mice were incubated with peptide stimulants at 37°C for 36 h, and then were harvested by centrifugation at 1,000 rpm for 20 min for IFN-γ ELISA kit assays (R&D).The optical density was read at 450 nm, and the IFN-γ concentrations were determined by a standard curve.
Virus titration
The bronchoalveolar wash was diluted fold10- serially, then inoculated on Madin Darby canine kidney (MDCK) cells, incubated at 37°C and examined for cytopathic effect 72 h later. The virus titer of each mouse was expressed as the 50% tissue culture infection dose (TCID50), and calculated by the Reed-Muench method. The virus titer in each experimental group was represented by the mean ± SD of the virus titer per ml of specimens from 3 mice in each group.Citation33,34
Statistics
The results of test groups were evaluated by one-way ANOVA with the Tukey multiple comparison. The survival rates of the mice in test and control groups were compared by using Kaplan Meier survival analysis. If P-value was less than 0.05, the difference was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Supplementary_Table_S1.docx
Download MS Word (15.9 KB)Funding
This study was supported by National Natural Science Foundation of China (Project No. 81172738, 81071346), Innovation Platform Open Fund of Hunan Provincial Education Department (11K010), National High Technology Research and Development Program of China (863 Program 2012AA02A401), Chinese State Key Project Specialized for Infectious Diseases (2013ZX10004003003) and Science and Technology Commission of Shanghai Municipality (12431901701).
References
- Altmüller A, Kunerl M, Müller K, Hinshaw VS, Fitch WM, Scholtissek C. Genetic relatedness of the nucleoprotein (NP) of recent swine, turkey, and human influenzaA virus (H1N1) isolates. Virus Res 1992; 22(1):79-87; PMID:1536092; http://dx.doi.org/10.1016/0168-1702(92)90091-M
- Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci 1985; 82(6):1785-9; PMID:3872457; http://dx.doi.org/10.1073/pnas.82.6.1785
- Influenza report 2006. ISBN 3-924774-51-X, http://www.influenzareport.com.
- Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis 2006; 12(1):48-54; PMID:16494717; http://dx.doi.org/10.3201/eid1201.051237
- Guo L, Zheng M, Ding Y, Li D, Yang Z, Wang H, Chen Q, Sui Z, Fang F, Chen Z. Protection against multiple influenza A subtypes by intranasal administration of recombinant nucleoprotein. Arch Virol 2010; 155(11):1765-75; PMID:20652335; http://dx.doi.org/10.1007/s00705-010-0756-3
- Roose K, Fiers W, Saelens X. Pandemic preparedness: toward a universal influenza vaccine. Drug News Perspect 2009; 22(2):80-92; PMID:19330167; http://dx.doi.org/10.1358/dnp.2009.22.2.1334451
- Chen Q, Kuang H, Wang H, Fang F, Yang Z, Zhang Z, Zhang X, Chen Z. Comparing the ability of a series of viral protein-expressing plasmid DNAs to protect against H5N1 influenza virus. Virus Genes 2009; 38:30-8; PMID:19067149; http://dx.doi.org/10.1007/s11262-008-0305-2
- Chen Z, Yoshikawa T, Kadowaki S, Hagiwara Y, Matsuo K, Asanuma H, Aizawa C, Kurata T, Tamura S. Protection and antibody responses in different strains of mouse immunized with plasmid DNAs encoding influenza virus haemagglutinin, neuraminidase and nucleoprotein. J Gen Virol 1999; 80(Pt 10):2559-64; PMID:10573147
- Zheng M, Luo J, Chen Z. Development of universal influenza vaccines based on influenza virus M and NP genes. Infection 2014; 42(2):251-62; PMID:24178189; http://dx.doi.org/10.1007/s15010-013-0546-4
- Kim TJ, Kim KH, Lee JI. Stimulation of mucosal and systemic antibody responses against recombinant transferrin-binding protein B of Actinobacillus pleuropneumoniae with chitosan after tracheal administration in piglets. J Vet Med Sci 2007; 69(5):535-9; PMID:17551229; http://dx.doi.org/10.1292/jvms.69.535
- Meng S, Liu Z, Xu L, Li L, Mei S, Bao L, Deng W, Li L, Lei R, Xie L, et al. Intranasal immunization with recombinant HA and mast cell activator C48/80 elicits protective immunity against 2009 pandemic H1N1 influenza in mice. PLoS One 2011; 6(5):e19863; PMID:21625486; http://dx.doi.org/10.1371/journal.pone.0019863
- McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med 2008; 14(5):536-41; PMID:18425129; http://dx.doi.org/10.1038/nm1757
- Powell TJ, Strutt T, Reome J, Hollenbaugh J, Roberts AD, Woodland DL, Swain SL, Dutton RW. Priming with Cold-Adapted Influenza A Does Not Prevent Infection but Elicits Long-Lived Protection against Supralethal Challenge with Heterosubtypic Virus. J Immunol 2007; 178:1030-8; PMID:17202366; http://dx.doi.org/10.4049/jimmunol.178.2.1030
- Wraith DC, Vessey AE, Askonas BA. Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol 1987; 68(2):433-40; PMID:3493324; http://dx.doi.org/10.1099/0022-1317-68-2-433
- LaMere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, Kaminski DA. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol 2011; 186(7):4331-9; PMID:21357542; http://dx.doi.org/10.4049/jimmunol.1003057
- Mazanec MB, Coudret CL, Fletcher DR. Intracellular Neutralization of Influenza Virus by Immunoglobulin A Anti-Hemagglutinin Monoclonal Antibodies. J Virol 1995; 69(2):1339-43; PMID:7815518
- Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci 1992; 89:6901-5; PMID:1323121; http://dx.doi.org/10.1073/pnas.89.15.6901
- Tamura SI, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of Influenza Virus Clearance by Thl Cells in the Nasal Site of Mice Immunized Intranasally with Adjuvant-Combined Recombinant Nucleoprotein. J Immunol 1996; 156:3892-900; PMID:8621928
- Mukhtar MM, Li S, Li W, Wan T, Mu Y, Wei W, Kang L, Rasool ST, Xiao Y, Zhu Y, et al. Single-chain intracellular antibodies inhibit influenza virus replication by disrupting interaction of proteins involved in viral replication and transcription. Int J Biochem Cell Biol 2009; 41(3):554-60; PMID:18687409; http://dx.doi.org/10.1016/j.biocel.2008.07.001
- Sui Z, Chen Q, Fang F, Zheng M, Chen Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine 2010; 28(48):7690-8; PMID:20870054; http://dx.doi.org/10.1016/j.vaccine.2010.09.019
- Sui Z, Chen Q, Fang F, Zheng M, Chen Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine 2010; 28(48):7690-8; PMID:20870054; http://dx.doi.org/10.1016/j.vaccine.2010.09.019
- Baltzly R, Buck JS, de Beer EJ. A family of long-acting depressors. J Am Chem Soc 1949; 71(4):1301-5; PMID:18128356; http://dx.doi.org/10.1021/ja01172a045
- Echtenacher B, Mannel DN. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 1996; 381:75-7; PMID:8609992; http://dx.doi.org/10.1038/381075a0
- Malaviya R, Ikeda T, Ross E. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-a. Nature 1996; 381:77-80; PMID:8609993; http://dx.doi.org/10.1038/381077a0
- McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy ofdraining lymph nodes during infection. Nat Immunol 2003; 4:1199-1205; PMID:14595438; http://dx.doi.org/10.1038/ni1005
- Xu L, Bao L, Li F, Lv Q, Yuan J, Xu Y, Deng W, Yao Y, Yu P, Qin C. Intranasal immunization of mice with inactivated virus and mast cell activator C48/80 elicits protective immunity against influenza H1 but not H5. Immunol Invest 2014; 43(3):224-35; PMID:24295504; http://dx.doi.org/10.3109/08820139.2013.859155
- Qiu M, Fang F, Chen Y, Wang H, Chen Q, Chang H, Wang F, Wang H, Zhang R, Chen Z. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem Biophys Res Commun 2006; 343(4):1124-31; PMID:16580631; http://dx.doi.org/10.1016/j.bbrc.2006.03.088
- Zheng L, Wang F, Yang Z, Chen J, Chang H, Chen Z. A single immunization with HA DNA vaccine by electroporation induces early protection against H5N1 avian influenza virus challenge in mice. BMC Infect Dis 2009; 9:17; PMID:19216752; http://dx.doi.org/10.1186/1471-2334-9-17
- Bradford MM. A rapid and sensitive method for the quantitation of microgran quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-54; PMID:942051; http://dx.doi.org/10.1016/0003-2697(76)90527-3
- Zhu Q, Chang H, Chen Y, Fang F, Xue C, Zhang F, Qiu M, Wang H, Wang B, Chen Z. Protection of inactivated influenza virus vaccine against lethal influenza virus infection in diabetic mice. Biochem Biophys Res Commun 2005; 329:87-9; PMID:15721277; http://dx.doi.org/10.1016/j.bbrc.2005.01.109
- Deng YJ, Yewdell W, Eisenlohr LC, et al. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J Immunol 1997; 158:1507-1515; PMID:9029084
- Gao XM, Liew FY, Tite JP. Identification and characterization of T helper epitopes in the nucleoprotein of influenza A virus. J Immunol 1989; 143:3007-3014; PMID:2478631
- Chen J, Zhang F, Fang F, Chang H, Chen Z. Vaccination with hemagglutinin or neuraminidase DNA protects BALB/c mice against influenza virus infection in presence of maternal antibody. BMC Infect Dis 2007; 7:118; PMID:17939857; http://dx.doi.org/10.1186/1471-2334-7-118
- Zhang F, Chen J, Fang F, Zhou Y, Wu J, Chang H, Zhang R, Wang F, Li X, Wang H, et al. Maternal immunization with both hemagglutinin- and neuraminidase-expressing DNAs provides an enhanced protection against a lethal influenza virus challenge in infant and adult mice. DNA Cell Biol 2005; 24:758-65; PMID:16274296; http://dx.doi.org/10.1089/dna.2005.24.758
