Abstract
Despite availability of effective rabies vaccines, India has the highest global mortality rate for rabies. Low socio-economic communities are most affected due to lack of awareness of the disease and poor compliance to post-exposure prophylactic regimens. Currently, the only approved intramuscular regimen for post-exposure prophylaxis (PEP) against rabies in India is the Essen regimen, which consists of 5 injections administered over 5 separate days in a period of one month. The high number of doses and clinical visits, however, are major reasons for non-compliance, and thus a shorter regimen would be beneficial. In a simulated PEP trial in healthy, adult subjects, this study evaluated whether purified chick embryo cell vaccine (PCECV), administered according to the WHO-recommended 4-dose/3 visit Zagreb vaccination regimen is of equal immunogenicity and safety as the standard Essen regimen in Indian subjects. Two hundred and 50 healthy adults were enrolled and randomized into a Zagreb or Essen group, each receiving PCECV according to their respective regimen. Blood samples were collected on Days 0, 7, 14 and 42 and analyzed using the rapid fluorescent focus inhibition test (RFFIT). By Day 14, all subjects across both groups attained rabies virus neutralizing antibody (RVNA) concentrations of ≥ 0.5IU/ml. The Zagreb regimen was then demonstrated to be immunologically non-inferior to the Essen regimen by Day 14, which was the primary endpoint of the study. No safety issues were noted and the occurrence of adverse events was similar in both groups (17% and 15%, respectively). NCT01365494. CTRI No.: CTRI/2011/07/001857
Introduction
Rabies is a fatal viral encephalomyelitis which, while incurable, can be prevented through effective pre- or post-exposure vaccination and timely administration of immunoglobulins.Citation1 Exposure to rabid animals is estimated to result in 60,000 deaths globally each year, primarily in African and Asian countries.Citation2 Of these, India has the highest annual mortality at over 20,000 deaths per year, mostly from poor or low-income communities.Citation2 Poverty, and lack of awareness of the disease or of the importance of initiating immediate post-exposure prophylactic (PEP) measures, are the primary reasons for the high incidence of rabies.Citation3
After the onset of clinical symptoms, rabies is almost invariably fatal with survival lasting only from a few days to weeks.Citation4,5 However, PEP treatment instituted as soon as possible after a rabies virus exposure (e.g. an animal bite) is highly effective in preventing the disease. In rabies-endemic countries such as India, dog bites are the primary source of human infection and thus PEP should be administered as soon as possible after an exposure.Citation3
Purified Chick Embryo Cell Vaccine (PCECV; Rabipur®, Novartis Vaccines) is a highly purified, potent and efficacious vaccine recommended by the World Health Organization (WHO) for both pre- and post-exposure prophylaxis against rabies.Citation6 It is one of 3 cell culture rabies vaccines currently available in India for pre- or post-exposure prophylaxis (intradermal or intramuscular); the other 2 being Purified Vero Cell Rabies Vaccine (PVRV), and Human Diploid Cell Rabies Vaccine (HDCV). At present, the only intramuscular (IM) regimen approved in India is the Essen (1–1–1–1–1) regimen, which is a schedule that consists of 5 IM injections of anti-rabies vaccines administered on Days 0, 3, 7, 14 and 28.Citation2,7 Unfortunately, despite the availability of effective rabies vaccines in both the government and private sector, rabies continues to claim lives in India.Citation7 The cost and duration of the PEP regimen frequently results in preventative interventions either not being adopted at all or not being completed.Citation3,8,9
The four-dose Zagreb (2–1–1) IM regimen (consisting of 2 doses on Day 0, followed by one dose each on Days 7 and 21) is an alternative vaccination regimen also recommended by the WHO that has been implemented in other countries for many years.Citation10–12 It involves administration of only 4 doses of rabies vaccine over 3 weeks, and thus it is relatively less expensive as well as more convenient than the Essen regimen.Citation13 These are both important factors to consider since one of the reasons for treatment failure is lack of compliance.Citation14 Should a shorter and equally effective immunization regimen be implemented, it can be expected that patient compliance would be significantly improved. To date, while the Zagreb (2–1–1) rabies regimen has already been evaluated in other countries.Citation10-12 its immunogenicity in an Indian population has not yet been established. Understanding a vaccine's safety and immunogenicity in different demographic populations is important, especially in India where the risk of contracting rabies is particularly high. In the present simulated post-exposure study, the aim was thus to verify that PCECV administered according to the Zagreb (4-dose) regimen is as immunogenic and safe as the Essen (5-dose) regimen in healthy Indian adults.
Results
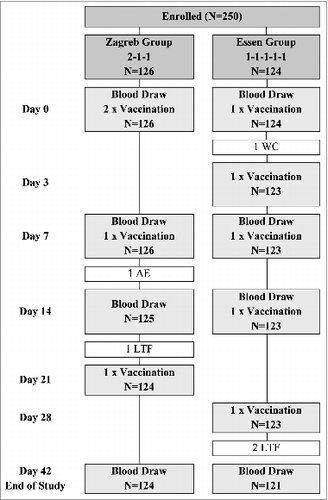
A total of 250 healthy Indian adults were enrolled at 3 anti-rabies clinics and randomized into 2 groups: a Zagreb and an Essen Group. At the time of enrolment, no significant differences in age, weight, or male/female ratio were apparent between the groups (see ). The majority (244/250; 98%) of subjects received their vaccinations and provided blood samples on the correct days (windows for blood draws: Days 7–8, 14–15, and 40–45). Six subjects (2%), however, had major protocol deviations: 2 in the Zagreb group and 4 in the Essen group. Of these, one subject (Essen group) failed to provide a blood sample within the Day 42 window (Days 40–45; they were thus excluded from immunogenicity analysis on this day only), one subject withdrew consent, 3 subjects were lost to follow up, and one subject withdrew due to an adverse event. Ultimately, 245 subjects completed the study (see for Subjects and Study Design).
Table 1. Demographics of enrolled subjects
Figure 1. Flow chart of study design for Groups Zagreb and Essen. Excluded subjects are noted in the white boxes. Abbreviations: AE, adverse event; LTF, lost to follow-up; WC, withdrew consent.
Immunogenicity analysis
The main aim of this study was to establish that PCECV (Rabipur®, Novartis Vaccines), administered according to the Zagreb (2–1–1) PEP regimen is immunologically non-inferior to the conventional Essen (1–1–1–1–1) regimen on Day 14.
Blood samples (approximately 5 mL) for immunogenicity analysis were collected from all available subjects on Days 0, 7, 14, and 42. RVNA concentrations were measured with the rapid fluorescent focus inhibition test (RFFIT)Citation15 and the results of this assay were expressed as: 1) RVNA geometric mean concentrations (GMCs), 2) geometric mean ratios (GMRs) i.e. the ratio of GMCs between the study groups, and 3) percentage of subjects attaining RVNA concentrations of ≥ 0.5 IU/ml (the universally accepted surrogate end-point for protection against rabies). This antibody titer, which is globally accepted, was established by the WHO as evidence of an immune response in humans against the rabies virus using a serum neutralization test with a standard challenge virus strain. It was selected based on early studies examining virus neutralization titers in human subjects vaccinated with cell culture vaccines at the Center for Disease Control and Prevention (CDC).Citation16,17
Antibody responses (GMCs) to PCECV for each group were measured on Days 0, 7, 14 and 42 () and the ratio of GMCs between the Zagreb and Essen Groups (GMRs) at each of these time points are shown in .
Table 2. RVNA GMCs and GMRs for the Zagreb and Essen Groups (95 % CI) on Days 0, 7, 14 and 42
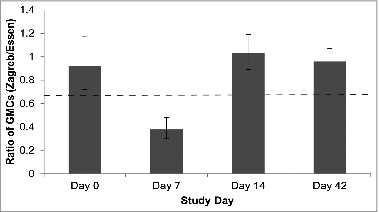
Figure 2. The ratio of GMCs between the Zagreb and Essen Groups (GMRs) on Days 0, 7, 14 and 42. Dashed line signifies threshold for statistical non-inferiority (GMR > 0.667).
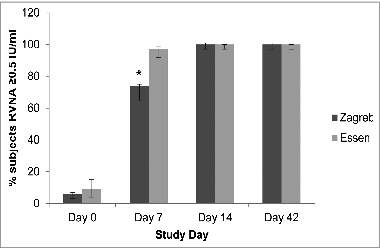
shows the percentages of subjects attaining RVNA concentrations (GMCs) ≥ 0.5 IU/mL on Days 0, 7, 14 and 42. On Day 7, a higher percentage of subjects in the Essen Group attained GMCs ≥ 0.5 IU/mL (97%) compared to the Zagreb Group (74%). On Days 14 and 42, however, 100% of subjects in both groups (Zagreb and Essen) attained GMCs ≥0.5 IU/mL. A few subjects in both groups (n = 8 [6%] and n = 11 [9%] of subjects in the Zagreb and Essen groups, respectively) exhibited RVNA concentrations ≥ 0.5 IU/mL on Day 0 (RVNA concentration range: 0.8–1.2 for the Zagreb group and 0.8–1.5 for the Essen group). The reason for this is unclear since subjects with prior history of rabies vaccination were excluded from the study.
On Day 0, the ratio of GMCs between the vaccine groups (Essen to Zagreb group) was 0.92, which confirmed that the baseline GMCs for each vaccine group were comparable (, ). On Day 7, the Essen-to-Zagreb group GMR, however, dipped to 0.38 (95% CI 0.3–0.48). Yet by Day 14, non-inferiority of the Zagreb compared to the Essen regimen was statistically established i.e., the lower limit of the 2-sided 95% CI for the GMRs between the groups (Zagreb/Essen) was greater than 0.667 (, ; GMR = 1.03; 95% CI 0.89–1.19). Since the 95% CI fell completely within the interval (0.667, 1.500), the 2 treatments were shown to be equivalent based on this non-inferiority margin. GMCs from Days 0 to Day 14 for each center were also documented (see Supplementary ) to determine whether there were any significant variations between different study sites. As can be seen in this table, the mean GMC in the Essen group was numerically higher at Center 3 on Day 7 while the mean GMC on Day 14 in this same group was numerically higher at Center 1. The baseline (Day 0) GMCs across centers and groups, however, were comparable. The reason for the numerical differences between centers on Days 7 and 14 is unclear.
Safety
All adverse events (AEs) were collected as unsolicited AEs (). Information on AEs was collected for 7 d following administration of each study vaccination or until the time of the next vaccination (whichever occurred sooner). No immediate systemic hypersensitivity reactions (within 30 minutes) were reported after any of the vaccination doses. Overall, AEs, whether considered related to the vaccine or not, were reported by 17% and 15% of subjects in the Zagreb and Essen Groups, respectively. Of these, possibly related AEs were reported by 4% and 11% of subjects in the Zagreb and Essen Groups, respectively.
Table 3. Summary of Possibly Related Adverse Events (AEs)
The most commonly reported possibly related AEs in both the Zagreb and Essen regimens were pain at the injection site (2% and 7% of subjects respectively) and fever (2% of subjects in each regimen). No severe AEs (SAEs) or deaths occurred in the study. However, one subject from the Essen regimen withdrew from the study on Day 9 due to reported AEs (chills, pain at injection site and fever).
Discussion
Rabies is a fatal neurological disease transmitted to humans primarily by infected dogs, and while there is no cure once clinical symptoms set in, the disease is preventable by PEP measures including vaccination against rabies. Cell culture PEP vaccinations are effective with an acceptable safety profile, and have been available in India since the 1980s.Citation1 For treatment to be most successful, however, compliance with the recommended immunisation regimen is critical. Unfortunately, due to lack of awareness or knowledge about rabies PEP, combined with the cost of vaccination and the need for repeated visits to a health clinic, rabies PEP vaccinations have been poorly adopted in India.Citation8,9 Consequently, India continues to have the highest annual worldwide mortality from rabies.Citation2,3,8 Ultimately, a simpler rabies vaccination regimen with fewer injections would be an enormous benefit. Currently, while both the 5-dose Essen immunization regimen and the 4-dose Zagreb regimen are recommended by the WHO for PEP against rabies,Citation2 only the Essen regimen is currently approved for IM administration in India.Citation7
The effectiveness of the 4-dose Zagreb regimen was first investigated by Vodopija et al in 1986.Citation18 In their report, they compared the immunogenicity of 4 different cell culture vaccines (including PCECV) administered post-exposure according to the Zagreb regimen. In their study, each PEP vaccine resulted in 100% subjects attaining high RVNA levels (≥ 0.5 IU/mL) by Day 14. This four-dose regimen was subsequently endorsed by the WHO in 1992.Citation19 Numerous studies over the years have served to verify that cell culture vaccines administered under the Zagreb regimen are immunogenic with an acceptable safety profile.Citation10-12,18,20-22 However, only a few have directly compared the immunogenicity and safety of the Zagreb vs. the Essen regimen.Citation12,18
The primary finding in the present study was that vaccination with PCECV according to the Zagreb regimen was equally as effective as the conventional Essen vaccination regimen in healthy Indian subjects. A minimum RVNA concentration of 0.5 IU/ml serum, measured by the RFFIT, is a WHO-recommended and widely accepted criterion indicating adequate immunity against rabies; and in healthy subjects this level is typically achieved by Day 14 of a PEP regimen.Citation2 A small percentage of subjects (6% and 9% in the Zagreb and Essen group, respectively) enrolled in this study already exhibited RVNA titers > 0.5 IU/ml at baseline (Day 0). Given that there was no history of prior exposure to a suspected rabid animal or vaccination against rabies, the reason for these baseline elevated RVNA titers is unclear.
Overall, however, on Day 14, the primary objective of the study was achieved by demonstrating that antibody responses in healthy subjects following administration of PCECV according to the Zagreb regimen were statistically non-inferior to those following the Essen regimen (RVNA titers ≥ 0.5 IU/ml in both groups). On Day 7, the rate of subjects achieving RVNA titers≥ 0.5 IU/ml was numerically lower in the Zagreb (74%) compared to the Essen (97%) group. However, the number of subjects attaining this level for the Zagreb Group on Day 7 in this study is similar to, or indeed higher than, previous studies at this same time point using the same regimen and vaccine.Citation12,18 While such comparisons can be informative, it should be noted that between-study antibody concentrations can only be considered as indicative, due to differences in population characteristics and laboratory variations. The results, nevertheless, are consistent with the time delay expected before an individual mounts an appropriate immune response to the rabies virus. Hence, the WHO recommends Rabies Immunoglobulins (RIG) to be administered immediately, and concurrently with PEP, following Category 3 exposures (single or multiple transdermal bites or scratches, licks on broken skin or contamination of mucous membranes with the animal's saliva) to offer protection during this initial period of post-exposure vaccination. Certain studies, however, have indicated that human RIG has a greater immunosuppressive effect on rabies vaccine-induced antibody formation when administered according to the Zagreb (2–1–1) than to the Essen (1–1–1–1–1) regimen.Citation10,22,23 And although debatable, one study has suggested that equine RIG be used in preference to human RIG when treating a patient according to the Zagreb regimen since it has less of an immunosuppressive effect.Citation23 Human RIG is also much more expensive and thus primarily used in industrialized countries.Citation2 In the present clinical trial, RIG was not administered to any of the subjects since the study population consisted of healthy, non-exposed individuals.
Ideally, the rabies PEP regimen should be as short as possible in order to maximize compliance. Researchers are therefore continuing to investigate new methods for simplifying rabies vaccination regimens. While evidence exists that even fewer vaccinations may be effective in preventing rabies,Citation20 and while shorter post-exposure regimens (e.g., Two–1) have been recently proposed,Citation24 more evidence is needed to establish their immunogenicity and safety. In the meantime, the Zagreb regimen is a WHO-approved IM regimen which has been used internationally for many years. In the present study, we provide the first head-to-head comparison in an Indian population demonstrating that PCECV administered according to the 4-dose regimen is comparable to the standard 5-dose Essen regimen in terms of both safety and immunogenicity. While this study provides robust short-term data on the Zagreb and Essen regimens, the present study was not designed to measure persistence of antibody concentrations over a long period of time. Children were also not included in the study because, although they are an “at risk” age group, they are also considered to be a vulnerable population by the ICH-GCP, and thus require special safeguards in clinical trials. Ultimately, however, adoption of the 4-dose/3-visit Zagreb PEP rabies immunization regimen as an additional IM vaccination regimen in India could potentially reduce the current rate of rabies mortality by improving patient compliance due to lower medical costs and fewer hospital visits.
Conclusion
To increase patient compliance and reduce mortality from rabies in India, a shorter but equally immunogenic PEP vaccination regimen would be desirable. In this study, we have demonstrated that the Zagreb 4-dose/3 visit rabies vaccination regimen is non inferior in terms of both immunogenicity and safety, to the conventional Essen 5-dose/5-visit regimen by Day 14, which was the primary endpoint of the study, as well as on Day 42, which was the subsequent and final time point for estimation of RVNA titers. The subjects attaining RVNA levels of≥ 0.5 IU/mL with both regimens was 100% on Days 14 and 42. Both the schedules were well tolerated and there were no unexpected, serious or severe adverse reactions reported.
Materials and Methods
Study design
This Phase IV, randomized, open-label study was conducted at 3 centers across India between July 2011 and November 2011 (Clinicaltrials.gov identifier: NCT01365494; CTRI number: CTRI/2011/07/001857) after obtaining approval from Institutional Ethics Committees. Before enrolment, written informed consent was obtained from all subjects. The study was conducted according to the Ethical Guidelines for Biomedical Research issued by the Indian Council of Medical Research, the International Conference on Harmonization (ICH) Harmonized Tripartite Guidelines for Good Clinical Practice (GCP), with applicable local regulations, and in accordance with the Declaration of Helsinki.
The main objective of the study was to establish that PCECV (Rabipur®, Novartis Vaccines), administered according to the Zagreb PEP regimen is non-inferior to the conventional Essen regimen. Antibody responses and the percentage of subjects attaining RVNA levels of ≥ 0.5 IU/mL with both regimens were recorded and compared on Days 0, 7, 14 and 42 (see ). Safety data were also collected throughout the study period, and reported as adverse events (AEs) or severe AEs (SAEs).
Subjects
Two hundred and 50 adults were enrolled in this study. Subjects were included if they were ≥ 18 y in age, in good health, available for all scheduled visits, and had provided written informed consent. Subjects were excluded if any of the following applied: previous receipt of a rabies vaccination; pregnancy or unwillingness to practice acceptable contraception during the study; egg protein allergy; hypersensitivity to neomycin or any other vaccine component; serious acute or chronic infectious disease or use of antibiotics that may impact the subject's safety and /or immunogenicity; fever (≥ 38.0°C) up to 3 d prior to the study; receipt of an antimalarial drug up to 2 months prior to the study; known/suspected immune system impairment (including HIV or HIV-related disease); use of immunosuppressant drugs within 6 months of the study; use of systemic or chronic high potency inhaled corticosteroids within 30 d of the study; receipt of any other vaccine up to 28 d prior to enrolment; receipt of blood, blood products and/or plasma derivatives or any parenteral immunoglobulin preparation within 12 weeks of the study; any behavioral, psychological, or cognitive impairment/disease that interfered with a subject's ability to participate in the study; progressive or severe neurologic disorder, seizure disorder or Guillian-Barré syndrome; individuals who were not able to comprehend and/or follow all study procedures; simultaneous participation in any other clinical trial within 30 d of the study; study personnel or their close family members; planned surgery during the study; any condition associated with prolonged bleeding; a malignancy (excluding nonmelanotic skin cancer) or lymphoproliferative disorder.
Vaccines and vaccinations
Rabipur® (PCECV; Novartis Vaccines, India) was administered into the deltoid region (IM). The lyophilized vaccine (batch number 2062, Chiron Behring Vaccines Private Limited, Ankleshwar, Gujarat, India; potency 5.71 IU/dose) was supplied in a vial with a separate ampoule of clear, colorless, sterile diluent (water for injection) and a sterile syringe for reconstitution before use. One mL of reconstituted vaccine (strain Flury LEP) was administered at any one time. Subjects were randomized into either the Zagreb or Essen Group in a 1:1 ratio. The Zagreb Group received the vaccine according to the Zagreb regimen (4 doses: 2 administered on Day 0 and one administered separately on Days 7 and 21) while the Essen Group received the vaccine according to the Essen regimen (5 doses: each administered separately on Days 0, 3, 7, 14, and 28).
Immunogenicity analysis
Approximately 5 mL of blood was collected from every subject on Days 0, 7, 14, and 42 for immunogenicity analysis prior to any vaccination. Serological evaluations were conducted using the RFFIT, as advocated by the WHO,Citation2 with some modifications.Citation15 Analyses were performed at the WHO Collaborating Center for Reference and Research on Rabies in the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India, as described by Kulkarni et al., 2013.Citation25
Safety analysis
All subjects who had received a minimum of one vaccination and who provided safety data were considered for safety analysis. Following vaccine administration, all subjects were observed for at least 30 minutes for any immediate reactions. AEs were then recorded daily in a diary card for 7 d following each vaccination or until the time of the next vaccination (whichever occurred sooner). Any AE, including SAEs, were recorded from the time of consent until Day 42 (study end). The severity of AEs were classified as mild, moderate, or severe by the investigator, and the relationship to the study vaccine was determined as not related, possibly related, or probably related. All AEs, regardless of severity or whether related to the study vaccine, were monitored until resolution. Solicited local and systemic reactions were not recorded.
Statistical analysis
Approximately 250 subjects were planned for this study to provide adequate power (90%) for evaluation of the primary objective, assuming a 10% dropout rate on Day 14. The full analysis set (FAS) comprised subjects who had received at least one vaccination and who provided at least one evaluable sample. The per protocol set (PPS) comprised all subjects who had received the vaccine correctly, provided an evaluable blood sample on Day 14, and had no major protocol violations.
Non-inferiority of the Zagreb regimen compared to the Essen regimen was determined using the PPS on Day 14. Success was considered to be achieved when the lower limit of the 2-ended 95% CIs on Day 14 for the GMRs between the groups (Zagreb/Essen) was greater than 0.667. The lower limits of the 2-ended 95% CIs for the GMRs were calculated as follows: an analysis of variance (ANOVA) model was utilized to compute the difference between the least square (LS) means of the log-transformed titers and the associated 2-sided CI (adjusting for center). GMRs and associated 2-sided 95% CIs were then calculated by exponentiating (base10) the corresponding log-transformed difference of LS means from the model and associated 2-sided 95% CIs. All statistical calculations were performed using nQuery Advisor 6.01 and the significance level was 0.025 (one-sided).
The percentage of subjects achieving RVNA levels of ≥0.5 IU/ml and GMCs on Days 0, 7, 14 and 42 were performed on the FAS and were analyzed descriptively. No formal statistical analyses were performed on these data.
Disclosure of Potential Conflicts of Interest
Khaleel Ahmed, Rekha Jonnalagedda, Hoshang Vakil, Chiranjiwi Bhusal, and Ashwani Kumar Arora are all permanent employees of Novartis Vaccines. BJ Mahendra, DH Ashwath Narayana, Sharad Agarkhedkar, HS Ravish, BR Harish, Shalaka Agarkhedkar, SN Madhusudana, and Ashwin Belludi have no conflicts of interest to declare.
Author Contributions
All authors were involved in the conduct of the study, acquisition, analysis and interpretation of data, development of the initial draft of the manuscript, review and revision of the manuscript, and approval of the final manuscript as submitted. KA, HV, CB, and AKA were also involved in the conception and design of the study. KA, CB and AKA were also involved in the statistical analysis and interpretation of the data.
Acknowledgments
The authors are grateful to all volunteers who participated in the clinical trial, to Dr. Yvonna Fisher-Jeffes (Novartis Vaccines) for her writing and editorial assistance in the preparation of this manuscript and to Allen Izu for his assistance in the biostatistical analysis of the data.
Funding
This trial was supported by funds provided by Novartis Vaccines.
References
- Nandi S, Kumar M. Development in Immunoprophylaxis against Rabies for Animals and Humans. Avicenna journal of medical biotechnology 2010; 2:3-21; PMID:23407587
- (WHO) WHO. Rabies Vaccines: WHO position paper. Weekly epidemiological record 2010; 32:309-20; PMID:18064757
- Menezes R. Rabies in India. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2008; 178:564-6; PMID:18299543; http://dx.doi.org/10.1503/cmaj.071488
- Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. Journal of neurovirology 2005; 11:93-100; PMID:15804967; http://dx.doi.org/10.1080/13550280590900409
- Gadre G, Satishchandra P, Mahadevan A, Suja MS, Madhusudana SN, Sundaram C, Shankar SK. Rabies viral encephalitis: clinical determinants in diagnosis with special reference to paralytic form. Journal of neurology, neurosurgery, and psychiatry 2010; 81:812-20; PMID:19965838; http://dx.doi.org/10.1136/jnnp.2009.185504
- WHO. WHO prequalified vaccines. 2012.
- Ministry of Health & Family Welfare GoI. National Guidelines for Rabies Prophylaxis and Intra-dermal Administration of Cell Culture Rabies Vaccines. 2007
- Ichhpujani RL, Chhabra M, Mittal V, Bhattacharya D, Singh J, Lal S. Knowledge, attitude and practices about animal bites and rabies in general community–a multi-centric study. The Journal of communicable diseases 2006; 38:355-61; PMID:17913213
- Baxter JM. One in a million, or one in thousand: What is the morbidity of rabies in India? Journal of global health 2012; 2:010303; PMID:23198128; http://dx.doi.org/10.7189/jogh.01.010303
- Chutivongse S, Wilde H, Fishbein DB, Baer GM, Hemachudha T. One-year study of the 2-1-1 intramuscular postexposure rabies vaccine regimen in 100 severely exposed Thai patients using rabies immune globulin and Vero cell rabies vaccine. Vaccine 1991; 9:573-6; PMID:1771970; http://dx.doi.org/10.1016/0264-410X(91)90244-Z
- Colnot F, Sureau P, Alexandre JL, Arnaudo JP, Hesse JY, Jeanmaire H. [Post-exposure antirabies vaccination. Early serological response to vaccine cultivated on VERO cells using a reduced 2-1-1 schedule]. Presse Med 1994; 23:1609-12; PMID:7831241
- Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Human vaccines 2011; 7:220-4; PMID:21311216; http://dx.doi.org/10.4161/hv.7.2.14003
- WHO. Rabies Vaccines: WHO Position Paper. 2010
- Sudarshan MK, Narayana DH, Madhusudana SN, Holla R, Ashwin BY, Gangaboraiah B, Ravish HS. Evaluation of a one week intradermal regimen for rabies post-exposure prophylaxis: results of a randomized, open label, active-controlled trial in healthy adult volunteers in India. Human vaccines & immunotherapeutics 2012; 8:1077-81; PMID:22699446; http://dx.doi.org/10.4161/hv.20471
- Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. Journal of biological standardization 1979; 7:213-20; PMID:387798; http://dx.doi.org/10.1016/S0092-1157(79)80024-4
- Moore SM, Hanlon CA. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS neglected tropical diseases 2010; 4:e595; PMID:20231877; http://dx.doi.org/10.1371/journal.pntd.0000595
- Wunner WH, Jackson AC. Rabies: Scientific Basis of the Disease and Its Management. Academic Press, 2010
- Vodopija I, Sureau P, Lafon M, Baklaic Z, Ljubicic M, Svjetlicic M, Smerdel S. An evaluation of second generation tissue culture rabies vaccines for use in man: a four-vaccine comparative immunogenicity study using a pre-exposure vaccination schedule and an abbreviated 2-1-1 postexposure schedule. Vaccine 1986; 4:245-8; PMID:3541428; http://dx.doi.org/10.1016/0264-410X(86)90138-6
- (WHO) WHO. WHO Expert Committee on Rabies. WHO Technical Report 1992: 1-84.
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett S, Levis R, Meltzer MI, Schaffner W, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009; 27:7141-8; PMID:19925944; http://dx.doi.org/10.1016/j.vaccine.2009.09.029
- Vodopija I, Baklaic Z, Vodopija R. Rabipur: a reliable vaccine for rabies protection. Vaccine 1999; 17:1739-41; PMID:10194832; http://dx.doi.org/10.1016/S0264-410X(98)00427-7
- Vodopija I, Sureau P, Smerdel S, Lafon M, Baklaic Z, Ljubicic M, Svjetlicic M. Interaction of rabies vaccine with human rabies immunoglobulin and reliability of a 2-1-1 schedule application for postexposure treatment. Vaccine 1988; 6:283-6; PMID:3420976; http://dx.doi.org/10.1016/0264-410X(88)90225-3
- Lang J, Simanjuntak GH, Soerjosembodo S, Koesharyono C. Suppressant effect of human or equine rabies immunoglobulins on the immunogenicity of post-exposure rabies vaccination under the 2-1-1 regimen: a field trial in Indonesia. MAS054 Clinical Investigator Group. Bulletin of the World Health Organization 1998; 76:491-5; PMID:9868840
- Huang G, Liu H, Tang Q, Yu P, Shen X, Zhang Y, Liu X, Cao Q, Fu C, Liu B, et al. Making rabies prophylaxis more economical: Immunogenicity and safety results from a preliminary study using a 2-1 intramuscular regimen in healthy volunteers. Human vaccines & immunotherapeutics 2013; 10:1, 114-19; PMID:24008819; http://dx.doi.org/10.4161/hv.26264
- Kulkarni PS, Sapru A, D'Costa PM, Pandit A, Madhusudana SN, Yajaman AB, Mangrule S, Gunale B, Bavdekar AR. Safety and immunogenicity of a new purified vero cell rabies vaccine (PVRV) administered by intramuscular and intradermal routes in healthy volunteers. Vaccine 2013; 31:2719-22; PMID:23583817; http://dx.doi.org/10.1016/j.vaccine.2013.03.050