Abstract
CSL362 is a humanized interleukin-3 (IL-3)-neutralizing monoclonal antibody with enhanced effector function that binds the α subunit of the IL-3 receptor (IL3Rα). The crystal structure of an IL3Rα:CSL362 complex shows that IL3Rα adopts “open” and “closed” conformations. CSL362 blocks IL-3 function through both IL3Rα conformations but via distinct and unexpected mechanisms.
Abbreviations
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| AML | = | acute myeloid leukemia |
| ALL | = | B-cell acute lymphoblastic leukemia |
| CART | = | chimeric antigen receptor-transduced T cells |
| CML | = | chronic myeloid leukemia |
| GMRα | = | granulocyte-macrophage colony-stimulating factor receptor α subunit |
| IL-3 | = | interleukin-3 |
| IL3Rα | = | IL-3 receptor α subunit |
| IL5Rα | = | IL-5 receptor α subunit |
| IL13Rα1 | = | interleukin-13 receptor α subunit 1 |
| IL13Rα2 | = | interleukin-13 receptor α subunit 2 |
| LSPC | = | leukemic stem and progenitor cell |
| MDS | = | myelodysplastic syndromes |
| NK | = | natural killer cell |
| NTD | = | N-terminal domain |
| TKI | = | tyrosine kinase inhibitor |
The cytokine interleukin-3 (IL-3) regulates survival, proliferation, differentiation, and activation of cells of the hemopoietic and immune system . IL-3 signals through a receptor comprising the IL-3 receptor α subunit (IL3Rα or CD123) and the β common (βc) subunit.Citation1,2 IL3Rα is overexpressed in a number of hematological malignancies including acute and chronic myeloid leukemia (AML and CML respectively), myelodysplastic syndromes (MDS), B-cell acute lymphoblastic leukemia (ALL), hairy cell leukemia, and Hodgkin's lymphoma. Compared to normal hemopoietic stem cells, IL3Rα overexpression is also observed in leukemic stem and progenitor cells (LSPCs) from AML, CML, and MDS that represent a minor population of cells that give rise to the bulk tumor, but are also considered therapy-resistant and responsible for disease relapse.Citation3,4 At least in the case of AML, IL3Rα expression is inversely correlated with overall survival.Citation5 Thus, IL3Rα represents an attractive therapeutic target for a number of hematological malignancies for which a range of agents are currently being developed, including chimeric antigen receptor-transduced T cells (CART), IL-3:diphtheria toxin conjugates, and the therapeutic monoclonal antibody CSL362.Citation6,7
Figure 1. Dual mechanism of action of CSL362-induced cell death. The interleukin-3 (IL-3) receptor α subunit (IL3Rα) is overexpressed on various leukemic cells, including leukemic stem and progenitor cells (LSPCs), and is associated with poor prognosis in acute myeloid leukemia (AML). (a) IL-3-mediated receptor signaling results in formation of a complex between IL3Rα and the β common (βc) receptor (modeled on the granulocyte-macrophage colony-stimulating factor receptor structureCitation2) that induces cell survival, differentiation, or proliferation. (b) CSL362 is a humanized anti-IL3Rα neutralizing monoclonal antibody that has enhanced effector function and is currently in a phase I clinical trial in patients with AML. Neutralization of IL-3 signaling in leukemic cells enhances their susceptibility to tyrosine kinase inhibitor (TKI)-induced cell death and reduces the survival of LSPCs. CSL362 neutralizes IL-3-induced signaling by preventing IL-3 binding to IL3Rα (left) and by preventing higher order signaling complex formation (right). (c) In addition to its neutralizing activity, CSL362 also has enhanced effector function that results in potent natural killer (NK)-cell mediated antibody-dependent cell-mediated cytotoxicity (ADCC) killing of target cells, including AML blasts, chronic myeloid leukemia (CML) blasts, and LSPCs.
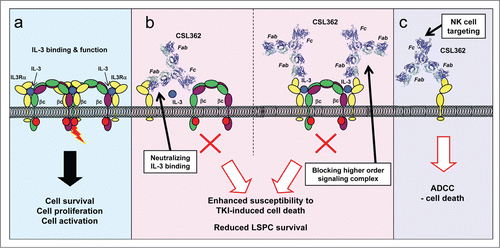
CSL362 is derived from the anti-IL-3 receptor blocking monoclonal antibody 7G3,Citation8 which was humanized, affinity optimized, and engineered via modifications in the Fc-domain to have a higher affinity for CD16, resulting in enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) activity (Fig. 1b and 1c).Citation7 CSL362 has been shown to deplete both CML and AML blasts by autologous natural killer (NK) cells in vitro.Citation7,9 Moreover, in mouse models of human AML and CML, CSL362 reduced engraftment of both human AML and CML stem cells.Citation7,9 The inhibitory effects on IL-3 contributed to reduced AML engraftment and reduced AML LSPC survival, and prevented IL-3–mediated rescue of dasatinib- or nilotinib-induced CML LSPC death. Importantly, however, CSL362 directly mediated killing of leukemic cells via NK cells.Citation7,9 These studies have prompted a phase I clinical trial of CSL362 in patients with AML (ClinicalTrial.gov identifier: NCT01632852).
We recently solved the crystal structure of a binary complex comprising a CSL362 Fab fragment bound to a soluble form of IL3Rα (IL3Rα:CSL362).Citation10 This study revealed that the N-terminal domain (NTD) of IL3Rα could adopt distinct conformations with respect to domains 2 and 3 (D2 and D3) of the receptor, with 2 non-identical forms of the IL3Rα:CSL362 complex observed within the crystal. The NTD of IL3Rα was positioned in either a “closed” conformation or a novel “open” conformation. The “closed” conformation closely resembled the receptor conformation adopted by related cytokine receptor subunits, including the interleukin-5 receptor α subunit IL5Rα and the interleukin-13 receptor α subunits IL13Rα1 and IL13Rα2, when bound to ligand. The main difference between the closed and open forms of the receptors was the angle between the NTD and D2, which showed a difference of approximately 40°. The open and closed forms of IL3Rα observed in the crystal structure likely represent the dynamic extremes of potential NTD conformations. A disulfide bond between C76 in the NTD and C194 in D2 and a hydrogen bond between S74 in the NTD and D196 in D2 place constraints on the degree to which the NTD can open, whereas interactions between A72 in the NTD and F198 in D2 provide a “doorstop effect” to limit further closing of the IL3Rα. Our data demonstrated that a likely consequence of the flexibility in the NTD is to allow subtle modulation of ligand binding and subsequent downstream signaling. IL5Rα, IL13Rα1, IL13Rα2, and the granulocyte-macrophage colony-stimulating factor receptor α subunit, GMRα, also have a flexible linker between the NTD and D2, suggesting a similar mechanism of receptor modulation.
Our crystal structure revealed that CSL362 binds exclusively to the IL3Rα NTD through interactions involving the BC, DE, and FG loops and strand D of the NTD.Citation8,10 Investigation of the functional IL-3 binding site in IL3Rα through homology modeling and mutagenesis studies indicated that the binding interactions of CSL362 and IL-3 with IL3Rα are largely non-overlapping, and prompted further analysis to understand how CSL362 is able to function as an antagonist of IL-3 function. In the closed IL3Rα complex, binding of CSL362 prevents IL-3 binding through steric interference whereas in the open IL3Rα complex CSL362 and IL-3 can potentially bind simultaneously. Using IL3Rα mutations that were proposed to force IL3Rα to adopt an “open-like” conformation, we showed that CSL362 and IL-3 can simultaneously bind to an IL-3 receptor complex where IL3Rα exists in an open-like conformation, but that the binding of CSL362 still blocks IL-3 receptor function.Citation10 On the basis of these results we proposed that IL-3 signaling arises through the initial assembly of a 2:2:2, IL-3:IL3Rα:βc hexamer complex that provides high-affinity IL-3 binding. Subsequent formation of a higher-order complex that requires interactions between hexamers allows βc-associated JAK2 activation and is essential for signal transduction. Such higher-order complex formation was first reported for the GM-CSF receptor and is potentially a common feature of the βc family of receptors.Citation2 Importantly, CSL362 can inhibit IL-3-induced receptor activation in either the classic closed form by directly blocking IL-3 binding, or in the novel open form by preventing higher order complex formation (Fig. 1b).
The dual mechanisms of CSL362-mediated IL-3 neutralization demonstrate quite distinct structural explanations that may be germane in understanding the blocking activity of other antibodies that block hormone receptor function.
Disclosure of Potential Conflicts of Interest
MWP and AFL are consultants for CSL Limited which is developing CSL362 and have received research support from CSL Limited. MPH and NJW are employees of CSL Limited.
Acknowledgments
This research was partly undertaken at the Australian Synchrotron, Victoria, and we acknowledge the technical assistance of the CSL Research Department.
Funding
This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia, from Cancer Australia and from the Australian Cancer Research Foundation. Funding from the Victorian Government Operational Infrastructure Support Scheme to St Vincent's Institute is acknowledged. MWP is an NHMRC Research Fellow and SEB is a Postdoctoral Fellow supported by the Leukaemia Foundation of Australia.
References
- Lopez AF, Hercus TR, Ekert P, Littler DR, Guthridge M, Thomas D, Ramshaw HS, Stomski F, Perugini M, D’Andrea R, et al. Molecular basis of cytokine receptor activation. IUBMB Life 2010; 62: 509-18; PMID:20540154; http://dx.doi.org/10.1002/iub.350
- Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. The GM-CSFIL-3IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 2012; 250: 277-302; PMID:23046136; http://dx.doi.org/10.1111/j.1600-065X.2012.01164.x
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000; 14: 1777-84; PMID:11021753; http://dx.doi.org/10.1038/sj.leu.2401903
- Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 2009; 5: 31-42; PMID:19570512; http://dx.doi.org/10.1016/j.stem.2009.04.018
- Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, Latagliata R, Mariani G, Rossini A, Battistini A, et al. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 2002; 100: 2980-8; PMID:12351411; http://dx.doi.org/10.1182/blood-2002-03-0852
- Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, Maltese F, Galimberti S, Lopez AF, Biondi A, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol 2013; 161: 389-401; PMID:23432359; http://dx.doi.org/10.1111/bjh.12282
- Busfield SJ, Biondo M, Wong M, Ramshaw HS, Lee EM, Ghosh S, Braley H, Panousis C, Roberts AW, He SZ, et al. Targeting of acute myeloid leukemia in vitro and in vivo with an anti-cd123 mab engineered for optimal ADCC. Leukemia 2014; 28: 2213-21; PMID:24705479; http://dx.doi.org/10.1038/leu.2014.128
- Sun Q, Woodcock JM, Rapoport A, Stomski FC, Korpelainen EI, Bagley CJ, Goodall GJ, Smith WB, Gamble JR, Vadas MA, et al. Monoclonal antibody 7G3 recognizes the N-terminal domain of the human interleukin-3 (IL-3) receptor a-chain and functions as a specific IL-3 receptor antagonist. Blood 1996; 87: 83-92; PMID:8547680
- Nievergall E, Ramshaw HS, Yong AS, Biondo M, Busfield SJ, Vairo G, Lopez AF, Hughes TP, White DL, Hiwase DK. Monoclonal antibody targeting of IL-3 receptor alpha with CSL362 effectively depletes CML progenitor and stem cells. Blood 2014; 123: 1218-28; PMID:24363400; http://dx.doi.org/10.1182/blood-2012-12-475194
- Broughton SE, Hercus TR, Hardy MP, McClure BJ, Nero TL, Dottore M, Huynh H, Braley H, Barry EF, Kan WL, et al. Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody. Cell Rep 2014; 8: 410-9; PMID:25043189; http://dx.doi.org/10.1016/j.celrep.2014.06.038
