Abstract
As light-based control of fundamental signaling pathways is becoming a reality, the field of optogenetics is rapidly moving beyond neuroscience. We have recently developed receptor tyrosine kinases that are activated by light and control cell proliferation, epithelial–mesenchymal transition, and angiogenic sprouting—cell behaviors central to cancer progression.
Abbreviations
| CRY2 | = | photolyase homology region of cryptochrome 2 |
| LOV | = | light oxygen voltage |
| RTK | = | receptor tyrosine kinase |
In George Lucas’ epic Star Wars saga, Jedi knights battle an evil empire with light sabers. Now, scientists have started to use light from lasers and light-emitting diodes to explore new concepts for striking back at one of humanity's most threatening foes, cancer.
The concept of optogenetics is based on genetically encoded effectors of cell signaling that can be rapidly and reversibly activated by light. Optogenetic approaches have their roots in neuroscience and date back more than a decade, when researchers first managed to control neuronal activity with light upon introduction of animal rhodopsin or microbial opsins into a variety of model organisms. Since then, optogenetics has transformed neuroscience through the dissection of neural circuitry and brain function in health and disease.Citation1 Over the last few years, the optogenetic toolkit has expanded dramatically. As a result, an increasing number of signaling pathways that are critical for cell fate decisions and hence play major roles in cancer development and progression became amenable to manipulation by light. For instance, optically activated variants of son of sevenless 1, Raf 1, Rho A, Rac 1, phosphoinositide 3 kinase p85α, and low-density lipoprotein receptor-related protein 6 (activating the Wnt pathway) have been developed and used to study cellular signaling events with an unprecedented degree of spatial and temporal precision.Citation2
In recent issues of The EMBO Journal,Citation3 Nature Communications,Citation4 and Chemistry & Biology,Citation5 collaborative work from our groups in Austria and work from the group of Won Do Heo in Korea independently describe Opto-RTKs, receptor tyrosine kinases (RTKs) that can be controlled with light. These reports demonstrate blue light-induced, spatio-temporally precise activation of several members of this crucial cell surface receptor family. Considering the fundamental role of RTKs in cancer development and angiogenesis and the clinical importance of RTK inhibitors, Opto-RTKs hold great promise for the field of oncology.
RTKs consist of an extracellular ligand-binding domain, a single-pass transmembrane domain, and an intracellular tyrosine kinase domain. In Opto-RTKs, the ligand-mediated dimerization that is required and sufficient for activation of many RTKs is replaced with light-induced dimerization. In all published Opto-RTKs, light-sensitive protein domains from diverse non-animal species were attached to the far C-terminus of the RTK, whereas the original extracellular domains were either retained or replaced by heterologous domains. Our group screened light oxygen voltage (LOV) domains of photoreceptors found in plants, bacteria, and fungi and identified 3 aureochrome LOV domains that are capable of activating the RTKs murine fibroblast growth factor receptor 1 (mFGFR1), human epidermal growth factor receptor, and human ret proto-oncogene3 (). The Heo laboratory used the photolyase homology region of cryptochrome 2 (CRY2) from Arabidopsis thaliana to drive the activation of the RTKs neurotrophin tyrosine kinase receptor type 1/2/3 (NTRK1/2/3, also known as tropomyosin-related kinase A/B/C, TrkA/B/C)Citation4 and human FGFR1.Citation5 The choice of light-sensing protein domains may have functional implications as the LOV domains form dimers,Citation6 whereas CRY2 has been shown to form oligomeric complexes.Citation7 Importantly, both systems demonstrate light-induced simultaneous activation of the mitogen activated protein kinase, phosphoinositide 3 kinase, and phospholipase Cγ pathways, as expected for canonical RTK signaling and in contrast to methods designed for activation of single pathways. Furthermore, no activation of signaling in the absence of light is observed in either of the two systems. We focused on the role of FGFR1 in malignant growth and demonstrated that light-induced activation of Opto-mFGFR1 was sufficient to quantitatively control cell behaviors that are directly relevant to cancer: enhanced proliferation and epithelial–mesenchymal transition of cancer cells, and sprouting of blood endothelial cells.
Figure 1. Recently published optically controlled receptor tyrosine kinases (Opto-RTKs) and potential applications in cellular oncology. (A) Design principles of the first Opto-RTKs recently described by our groups in Austria and the group in Korea and accessible cellular functions. Manipulated RTKs include murine/human fibroblast growth factor receptor 1 (m/hFGFR1), human epidermal growth factor receptor (hEGFR), human ret proto-oncogene (hRET), and tropomyosin-related kinase A/B/C (TrkA/B/C). Activation was achieved through attaching light oxygen voltage (LOV) domains of V. frigida aureochrome 1 (VfAU1) or O. danica/N. gaditana putative aureochrome 1 (Od/NgPA1) to the intracellular domains (ID) of the receptors. Extracellular (ED) and transmembrane domains (TD) were either retained or removed. Fluorescent proteins such as mCitrine (Ci) can be further added to the protein. (B) Opto-RTKs can be used to test the effects of temporally or spatially defined signaling patterns on cancer-related functions. By (co-)expressing Opto-RTKs that respond to light of different color (depicted in blue and red) in specific cell types (e.g., epithelial cells, fibroblasts, endothelial cells, immune cells), an additional level of control can be achieved. Hallmark characteristics linked to cell functions that were manipulated by light via Opto-RTKs in the recent publications are highlighted.
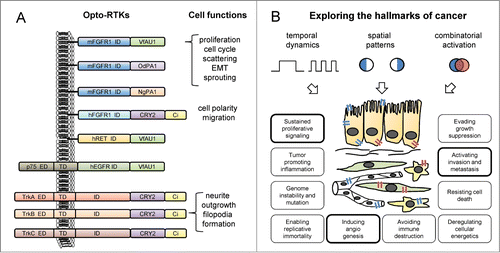
Using these newly-developed tools, researchers can now probe the hallmarks of cancer in new ways to obtain answers to long-standing questions.Citation8 One focus may lie in decoding the dynamic intracellular wiring of signaling pathways and understanding its link to functional changes in cell behavior. Repetitive short activation of cellular signaling may result in fundamentally different outcomes compared to prolonged activation.Citation9 Also, we have created variants of Opto-mFGFR1 with mutations in intracellular tyrosine residues that allow us to restrict activation patterns to specific combinations of downstream effectors. The overwhelming evidence for RTK hyperactivation during all stages of cancer contrasts with the seemingly paradoxical experience of many researchers that it can be difficult to overexpress RTKs in untransformed cells and even in fully malignant cancer cells without triggering apoptosis. Opto-RTKs may enable detailed studies of pathway-specific temporal and spatial dose-effect relationships between RTK activation and cell fates ranging from proliferation and migration to differentiation, senescence, and apoptosis. illustrates possibilities for spatially or temporally restricted activation of RTKs in specific loci of living cells, organotypic co-cultures, or model organisms. Since aberrant RTK signals contribute to most functional hallmarks of cancer, these experiments, combined with rapid and sensitive read-outs of cell signaling and behavior, will lead to a new understanding of key events underlying cancer in real-time and with high resolution. In high throughput formats, similar experiments may significantly improve and facilitate drug development. We expect that further advances in the field will create optically controlled variants of additional RTKs that can also be activated either simultaneously or separately (e.g., by light of different colors), and will enable patterned programming of cell functions and differentiation states with far-reaching implications in stem cell research and regenerative medicine. Future studies will profit from key enabling technologies that have been established in neuroscience. These include in vivo illumination technology, ranging from surgical implants to transdermal illumination, and 2-photon excitation approaches.Citation10
There may still be some way to go until these new tools ultimately benefit applied research and cancer treatment. Yet, analogous to developments in neuroscience, subsequent episodes in which researchers use Opto-RTKs for new discoveries in the field of oncology will soon follow.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci 2011; 34:389-412; PMID:21692661; http://dx.doi.org/ 10.1146/ annurev-neuro-061010-113817.
- Tischer D, Weiner OD. Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol 2014; 15:551-8; PMID:25027655; http://dx.doi.org/10.1038/nrm3837
- Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, Ingles-Prieto A, Janovjak H. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J 2014; 33:1713-26; PMID:24986882; http://dx.doi.org/ 15252/embj.201387695
- Chang KY, Woo D, Jung H, Lee S, Kim S, Won J, Kyung T, Park H, Kim N, Yang HW, et al. Light-inducible receptor tyrosine kinases that regulate neurotrophin signalling. Nat Commun 2014; 5:4057; PMID:24894073; http://dx.doi.org/10.1038/ncomms5057
- Kim N, Kim JM, Lee M, Kim CY, Chang KY, Heo WD. Spatiotemporal Control of Fibroblast Growth Factor Receptor Signals by Blue Light. Chem Biol 2014; 21:903-12; PMID:24981772; http://dx.doi.org/10.1016/j.chembiol.2014.05.013
- Toyooka T, Hisatomi O, Takahashi F, Kataoka H, Terazima M. Photoreactions of aureochrome-1. Biophys J 2011; 100:2801-9; PMID:21641326; http://dx.doi.org/10.1016/j.bpj.2011.02.043
- Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods 2013; 10:249-52; PMID:23377377; http://dx.doi.org/10.1038/nmeth.2360
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Brandman O, Meyer T. Feedback loops shape cellular signals in space and time. Science 2008; 322:390-5; PMID:18927383; http://dx.doi.org/10.1126/science.1160617
- Papagiakoumou E. Optical developments for optogenetics. Biol Cell 2013; 105:443-64; PMID:23782010; http://dx.doi.org/10.1111/boc.201200087
