Abstract
In most animals, during oocyte fertilization the spermatozoon provides DNA and centrioles together with some cytoplasm and organelles, but paternal mitochondria are generally eliminated in the embryo. Using the model animal C. elegans we have shown that paternal organelle degradation is dependent on the formation of autophagosomes a few minutes after fertilization. This macroautophagic process is preceded by an active ubiquitination of some spermatozoon-inherited organelles. Analysis of fertilized mouse embryos suggests that this autophagy event is evolutionarily conserved.
Keywords: :
Over the past years, C. elegans has been a powerful experimental model to explore the functions of autophagy in a whole animal and particularly during development. As a genetically tractable system, it contributed to unraveling the mechanisms of autophagy regulation, but also to better understanding various physiological functions of autophagy at the cellular and systemic levels. Studies in the nematode revealed a major role for autophagy in life-span extension, stress response, cell size regulation, cell survival and death, and neurotransmitter receptor trafficking as well as in the degradation of aggregate-prone proteins including the tissue-specific and physiological removal of maternally-inherited cell lineage determinants in the worm embryo. We used the C. elegans embryo to analyze the potential function of autophagy in the first minutes of development following fertilization.
Recent findings showed that autophagy is activated shortly after fertilization and is essential for pre-implantation development of mouse embryos. This has been proposed to participate in the degradation of maternal inherited components that are incompatible with embryo development.
In sexual reproduction, gamete fusion leads to the combination of two nuclear genomes. However, the contribution of the spermatozoids is not restricted to its DNA and associated centrioles, but also comprises several cytoplasmic components and organelles. Among them, the mitochondria are of particular interest because, in most animal species, the mitochondrial heredity is uniparental and strictly maternal, transmitted through the oocyte cytoplasm contribution. This relies on an unknown mechanism insuring the degradation of paternal mitochondria and their DNA, thus limiting the presence of damaged mitochondria and the risk of heteroplasmy. In mammals, ubiquitination of paternal mitochondria appears to be a hallmark for their selective degradation involving the lysosomal pathway. Proteasomal and autophagic degradation have been both proposed to participate in the removal of paternal mitochondria after fertilization, but strong experimental evidence was still lacking.
Fertilization of C. elegans oocytes occurs when they pass through the spermatheca, the organ where spermatozoa are stocked. C. elegans spermatozoa have no flagellum and contain mitochondria as well as nematode-specific membranous organelles underneath their plasma membrane (). Components of the membranous organelles were known to enter the ooplasm, but the behavior of spermatozoon mitochondria and their genome has not been previously described. Nevertheless, genetic experiments clearly demonstrated that paternal mitochondrial DNA is not transmitted to the offspring, indicating that C. elegans is therefore a good model to study the mechanism insuring uniparental mitochondrial DNA transmission and more generally to identify the mechanisms of the degradation of spermatozoa-inherited structures.
Figure 1. Transmission electron micrographs of a C. elegans spermatozoid (A) showing its DNA, membranous organelles (MO) and mitochondria (mt) prior to fertilization, and of the cortical area of a two-cell stage embryo (B) revealing the presence of autophagosomes around mitochondria. The white and black arrows indicate the limiting double membrane. Scale bars are 500 nm in (A and B). (C and D) Schematic view of the allophagy process. After fertilization, spermatozoon-inherited mitochondria and membranous organelles are engulfed in autophagosomes and degraded during the first zygote divisions (C). Interfering with autophagosome formation or fusion with lysosomes (not described) stabilizes spermatozoon-inherited organelles (D). (E) The entry of spermatozoid organelles in the mouse oocyte at fertilization induces ubiquitination and recruitment of autophagy markers (LC3) around the flagellum mid-piece still attached to the nuclear DNA. Scale bar is 5 μm in (E). (B) is adapted from Figure 1E, and (E) is adapted from Figures S5F and S5H of Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Science 2011; 334:1144–7.
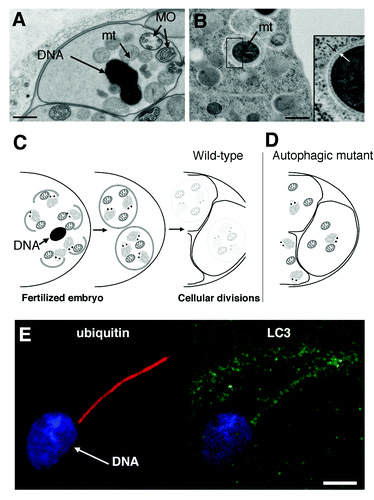
Using antibodies directed against LGG-1 and LGG-2, the two LC3 homologs in worm, we analyzed the presence of autophagosomes immediately after fertilization. While the mature spermatozoids are devoid of any staining, we observed a prominent punctate staining at the posterior pole of the newly fertilized embryo in close vicinity to the male pronucleus. To analyze in vivo the dynamics of autophagosome formation, we then generated a transgenic strain expressing GFP::LGG-2 under its own promoter and observed the presence of autophagosomes 20 min after fertilization, when the embryos proceed through the second meiotic division. We also found that LGG-1 and LGG-2 are detected as a diffuse cytoplasmic signal in the oocytes consistent with the fact that proteins are maternally inherited. Altogether, our results suggested that fertilization induces the formation of autophagosome-like structures, which could be involved in autophagic degradation of sperm-inherited components. We also demonstrated that both the nematode-specific membranous organelles and the spermatozoa mitochondria colocalize with autophagosome-like structures within the fertilized embryo, but disappear during the first cell divisions suggesting a quick degradation process. The presence of autophagosomes containing mitochondria was confirmed by transmission electron microscopy analysis of early embryos ().
In a second series of experiments, we inactivated the autophagy pathway in order to test whether it is involved in the degradation of these organelles. Both an lgg-1 mutant, as well as a combined RNAi depletion of LGG-1 and LGG-2, were used to interfere with autophagosome formation. This led to the stabilization of the membranous organelles, and the paternal mitochondria as well as their DNA during embryonic development. We confirmed that this degradative system involves a macroautophagy process by depleting two other components of the pathway, ATG-7 and RAB-7. For both proteins, an RNAi depletion also led to the stabilization of membranous organelles, supporting the involvement of a canonical autophagic pathway. While LGG-1 appears absolutely required for this degradation, the respective contributions of LGG-1 and LGG-2 remain unclear. We propose the name allophagy for the process of the degradation of sperm components by macroautophagy ().
What is the signal for the degradation of sperm-inherited organelles? Polyubiquitination of paternal mitochondria was previously proposed as the signal for their degradation in mouse embryos. Interestingly, in worms we observed that the MO but not the mitochondria were already ubiquitinated in the sperm and then further ubiquitinated after their entry in the oocyte. Using oocytes expressing GFP-ubiquitin, we confirmed that within a few minutes after their entry, ubiquitination of the MO was achieved. Interestingly, the ubiquitination appears to be both K63-linked and K48-linked suggesting that while K63-linked ubiquitin chains could target the MOs to autophagy, a contribution from proteasome-mediated degradation cannot be excluded. Autophagy has nevertheless a major role in their degradation since MO are largely remaining in autophagy-deficient embryos. While ubiquitination of MO is likely the signal for their degradation, the signal responsible for the degradation of mitochondria remains totally unknown. It will also be interesting to test whether this mitophagy is dependent on a strict targeting system, is a general mechanism for the elimination of depolarized mitochondria, or is a side effect of MO targeting.
We finally asked the question whether allophagy could be conserved in mammals, and analyzed the localization of LC3, GABARAP, p62 and K63-ubiquitin in fertilized mouse oocytes. The localization of these markers around the mid-piece of spermatozoa following their entry into the ooplasm strongly suggests that this autophagic response could also exist in mice and contribute to the degradation of paternal-inherited components including the mitochondria ().
This discovery gives us a unique physiological experimental system to study the mechanism of autophagosome formation, and deciphering the mechanisms of allophagy could have implications for animal cloning or human medically assisted reproduction.