Abstract
Our laboratory has been investigating for some time the nature of the response of T lymphocytes in autoimmunity in the reactions against self-proteins that result in a number of diseases, such as type 1 diabetes, multiple sclerosis, rheumatoid arthritis (RA) and others. T cells recognize peptides generated from proteins that are processed by antigen-presenting cells (APC). The peptides may derive from exogenous proteins or from the normal catabolism of self-proteins. The peptides complexed to major histocompatibility complex (MHC) molecules constitute the chemical entity that is engaged by the antigen-receptor of T cells. An important hypothesis postulates that self-peptides that suffer post-translational modifications in the APC may form neo-antigens that are recognized by the immune system and form the target of autoimmunity. Our interest in citrullination in the context of antigen processing and presentation stemmed from studies suggesting that an immune response to citrullinated self-peptides may be involved in autoimmunity. In a first publication, we found T cells that specifically recognized citrullinated peptides after immunization of inbred mice with standard foreign proteins. We used the small protein hen-egg white lysozyme. These T cells only recognized the citrullinated peptide and not the unmodified one, thus proving that a neo-epitope had been created by this modification. But how this modification took place was not known. Our recent report describes a central role for autophagy in citrullination of peptides by APC.
APC are represented by a group of cells that express the MHC molecules involved in interaction with CD4 T cells, the central cells that initiated autoimmune responses. The CD4 T cells recognize the class II-MHC molecules expressed in dendritic cells (DC) and macrophages, two cells that form part of the phagocytic lineage, as well as in B cells. DC and macrophages present citrullinated peptides as well as unmodified peptides from lysozyme. These cells have a constitutive level of autophagy. However, in contrast to phagocytic cells, B cells only present the unmodified peptide and not the citrullinated one. B cells have to be stimulated in order to generate citrullinated peptides. In B cell lines, stressing the cells, by brief serum starvation, induces autophagy and also induces presentation of citrullinated peptides. This is inhibited by either treatment with 3-methyladenine (3MA) or inhibition of ATG5 expression, manipulations that do not alter the presentation of unmodified peptides. These changes are not associated with alterations in expression of peptidylarginine deiminase (PAD), the enzyme involved in the conversion of arginine to citrulline.
Importantly, PAD activity is detected in purified autophagosomes. In primary B cells, B cell receptor (BCR) engagement by either cognate antigen or anti-immunoglobulin antibodies leads to induction of autophagy and presentation of citrullinated peptides, both inhibited by 3MA. Finally, after BCR engagement, we observed colocalization between internalized BCR, MHC and LC3. In brief, by pharmacological and genetic manipulations we concluded that there are two separate functional processing events (), one, that gives rise to unmodified peptides bound to class II-MHC molecules; and a second one giving rise to the citrullinated peptide bound to the MHC molecule. Only this second event depends on autophagy.
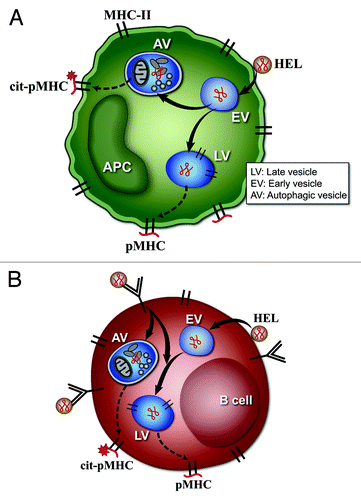
Figure 1. Model of citrullination by (A) DC and macrophages, or (B) B cells. (A and B) represent the possible scenarios taking place in APC that result in citrullination of peptide-MHC complexes (pMHC). (A) represents the pathways in DC or macrophages. These cells undergo constitutive autophagy and present the citrullinated peptides after the protein antigen comes into contact with the autophagy vesicles (AV). In (A), the protein antigen lysozyme (HEL) binds to the plasma membrane, and traffics to early endosomal vesicles (EV). From the EV it follows two pathways. In one it is taken to late vesicles (LV), processed to peptides and presented at the cell surface as a pMHC complex. In the second pathway, the protein is taken to an autophagic vesicle (AV) where it is processed to peptides, some of which are citrullinated and presented at the cell surface (cit-pMHC). (B) represents the pathways in B cells. Citrullination in B cells occurs only after autophagy is induced by engagement by antigen of the B cell receptor, shown as an immunoglobulin (Ig) molecule on the cell membrane. Receptor engagement leads to striking subcellular changes that lead to colocalization of AV and antigen-processing compartments, and presentation of citrullinated peptides. (B) shows that the antigen HEL can enter the B cell directly after interaction with plasma membrane, and is taken to EV and LV, to generate unmodified pMHC complexes. However, when HEL is bound to the surface Ig molecules, autophagy is induced, and some of the HEL-Ig complex then traffics to the AV to be processed, and generates the cit-pMHC complex.
The evidence linking autophagy to citrullination of antigen by APC raises several intriguing points related to autoimmunity. Changes in the tissue environment may affect the local APC to induce autophagy and with it citrullination, creating a substrate for autoreactivity. One of the strongest disease associations with citrullination is the disease RA. In RA, autoantibodies to citrullinated proteins are found, and serve as a marker of disease. Several environmental conditions such as smoking or infection are associated with RA. Autophagy in the APC may be the common feature of several factors, i.e., stressed conditions from smoking, joint injury, infection that may drive the adaptive responses to citrullinated self-proteins. There are two other issues to consider in the linkage of RA with autophagy and citrullination. First, a number of MHC alleles predispose to the disease, having in common the presence of positively-charged amino acids at the P4 peptide-binding pocket of the MHC. Peptides that show a neutral citrulline residue rather than a positively charged one are favored to bind to these allelic forms. The second issue has to do with our findings that BCR engagement by antibody induces presentation of citrullinated peptides. It has been known since the 1940s that patients with RA have auto-antibodies against autologous IgG and to a lesser extent to IgM. The combination of the presence of these autoantibodies, named rheumatoid factors (RF), and appropriate HLA alleles could constitute conditions that induce presentation of citrullinated self-peptides and potentially breach unresponsiveness to self-proteins, leading to the initiation or enhancement of pathological adaptive responses. BCR-induced presentation of citrullinated peptides may be the missing piece linking RF to RA. Finally, it is important to note that citrullination in the autophagosome may favor catabolism of the proteins, as charged residues of the proteins are eliminated. Regardless, citrullination needs to be considered as a biochemical marker of autophagy.
Acknowledgments
This research was supported by National Institutes of Health grants AI022033 and AI 024742.