Abstract
Premenopausal women have better survival than men after intracerebral hemorrhage, which is associated with iron overproduction and autophagy induction. To examine the participation of neuronal autophagy and estrogen receptor α (ERα) in the E2–mediated protection, PC12 neurons treated with Atg7 (autophagy-related protein 7) siRNA, rapamycin (an autophagy inducer), or Erα siRNA were applied. To study whether autophagy involves in β-estradiol 3-benzoate (E2)-mediated neuroprotection against iron-induced striatal injury, castration and E2 capsule implantation were performed at 2 weeks and 24 h, respectively, before ferrous citrate (FC) infusion into the caudate nucleus (CN) of Sprague Dawley male and female rats. Furthermore, the role of neuronal autophagy in the sex difference of FC-induced CN injury was confirmed by using conditional knockout Atg7 in dopamine receptor 2 (DRD2)-containing neurons in mice. The results showed that the suppression of FC-induced autophagy by E2 was abolished by Erα siRNA preincubation. Atg7 silencing simulates and rapamycin diminishes E2-mediated neuroprotection against FC-induced neurotoxicity. In vivo, FC induced a lower degree of autophagy, autophagic cell death, injury severity, histological lesion and behavioral deficit in female rats than in males. E2 implantation decreased the levels of both FC-induced autophagy and injury in ovariectomized rats. Moreover, the sex difference of FC-induced CN injury was diminished in Atg7 knockout mice. Thus, suppression of autophagy by E2 via ERα contributes to less severity of iron-induced brain injury in females than in male. This finding opens up the prospect for a therapeutic strategy targeting autophagic inhibition for patients suffering from intracerebral iron overload.
Introduction
Intracerebral hemorrhage (ICH) accounts for about 10–30% of stroke and the ICH 30-d mortality rate is higher than 50%.Citation1 In the brain of surviving patients, the overproduction of iron complexes because of the lysis of red blood cells leads to free radical formation, oxidative chain reactions, and thus neurotoxicity/brain injury.Citation1 Therefore, even after removing the blood clot, delayed onset brain injury remains a major complication in patients suffering from ICH.Citation2 Premenopausal women have better survival and functional recovery than men after ICH.Citation3,Citation4 Female rodents tolerate ICH better than males with less edema, a faster recovery from behavioral deficits, and better outcome.Citation5 Moreover, estradiol (E2) has been shown to confer protection in experimental ICHCitation6 and iron-induced brain injury.Citation7 However, the underlying mechanism of the sex-dimorphic pathogenesis after iron overload remains unclear.
Autophagy is an essential recycling system for maintaining physiological homeostasis.Citation8 However, iron-induced autophagy may contribute to brain injury after ICH in some conditions.Citation9 Autophagy is enhanced by oxidative stress, and inhibited by mechanistic target of rapamycin kinase (MTOR).Citation10 E2 exhibits an antioxidant effect,Citation11 possibly through the activation of MTOR via ERα.Citation12 Accordingly, we hypothesize that E2 protects brain from iron-induced injury in females via inhibiting iron-induced autophagy through an ERα-dependent mechanism.
To test this hypothesis, the participation of neuronal autophagy or ERα in E2-mediated protection was elucidated in vitro. Effects of E2 on the FC-induced striatal injury and autophagy were also examined in vivo. Spectrin breakdown products (SBDPs), fragments SBDP 145/150, were used as a marker of injury severity in both in vivo and in vitro studies, because α-II spectrin is a structural protein abundant in neurons and is cleaved into fragments by proteases involved in cell death.Citation13 During autophagy, a cytosolic form of microtubule-associated protein 1 light chain 3 (LC3-I) is conjugated with phosphatidylethanolamine to form lipidated LC3-II, which aggregates on the autophagosomal membrane, both LC3 aggregation and the level of LC3-II were examined as markers of autophagy. To investigate the involvement of neuronal autophagy in protection conferred by E2, loss or gain of autophagic function was obtained in vitro by silencing of Atg7 (an essential gene for autophagosome formation) and preincubation with rapamycin (an autophagy inducer), respectively. Furthermore, mice with conditional knockout of Atg7 in DRD2-containing neurons, which are abundant in striatum,Citation14 were used to confirm the participation of neuronal autophagy in the sex difference of FC-induced injury.
Results
Role of autophagy modulation in the E2-mediated protective effect on FC-induced neurotoxicity in PC12 cells
Rat PC12 cells were differentiated into neurons by NGF induction in vitro to examine whether autophagy participates in the E2-mediated protection against the neurotoxicity induced by FC. Two days after NGF treatment, the cells extended neuron-like process. After FC exposure, part of the cells underwent round up, while pretreatment of E2 significantly decreased the number of cells with round-up morphology. The results showed that FC exposure increased levels of LC3-II by 48 ± 6.8% (p < 0.05) and SBDP 145/150 kD by 97 ± 13% (p < 0.01), and preincubation with E2 (10 nmol/l) decreased the FC-induced autophagy () by 45% ± 5.8% (p < 0.01), cleavage of α-II spectrin () by 53 ± 7.2% (p < 0.01) and cell death () by 40 ± 2.1% (p < 0.01). Moreover, FC induced an increase in the number of TUNEL(+) BECN1 immunoreactive cells reflecting autophagic cell death, whereas E2 preincubation decreased the incidence of FC-induced autophagic cell death ().
Figure 1. E2 pretreatment decreases FC-induced autophagy, injury, autophagic cell death and neurotoxicity in PC12 cells. (A) Autophagy was induced after FC exposure and E2 significantly decreased FC-induced autophagy. (B) FC induced the neuronal injury and E2 significantly decreased the FC-induced neuronal injury. (C) E2 significantly decreased FC-induced autophagic cell death. Cells were stained by TUNEL and BECN1 for DNA damage (green) and autophagy (red), respectively. Nuclei were stained by DAPI. The TUNEL(+) Beclin 1 immunoreactive cells with an intact nucleus in PC12 cells pretreated with or without E2 before FC exposure depict an index of autophagic cell death as indicated by arrows in the merged view. (D) FC increased the number of dead cells and E2 significantly increased the number of living cells after FC exposure. Data are expressed as means ± SD (n = 6), *p < 0.05, p** < 0.01.
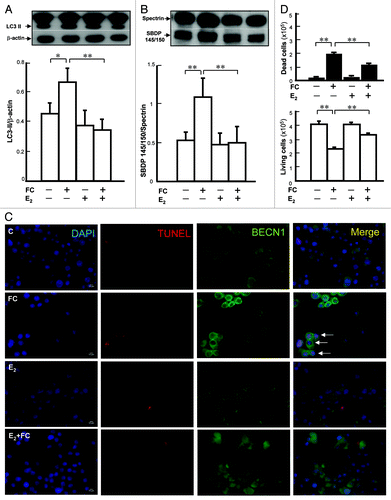
Atg7 siRNA was used to investigate whether the suppression of autophagy by E2 might mediate the protection against FC-induced neurotoxicity conferred by E2. As shown in , the degree of FC-induced LC3 lipidation was decreased by 42 ± 2.3% (p < 0.05), at 48 h after silencing Atg7. Pretreatment of Atg7 siRNA per se simulated the protective effect of E2 on FC-induced neurotoxicity (). Pretreatment with the MTOR inhibitor rapamycin, which enhances autophagy, at a concentration of 25 nmol/l for 29 h increased the basal level of LC3-II () by 200 ± 2.4% (p < 0.01) and totally eliminated the protective effect of E2 on FC-induced cytotoxicity ().
Figure 2. Involvement of autophagy in E2-mediated neuroprotection against FC-induced neurotoxicity in PC12 cells. (A) Atg7 silencing decreased the levels of both constitutive and FC-induced autophagy. The differentiated PC12 cells were pretreated with 100 nM Atg7 siRNA or nontargeted siRNA for 24 h followed by E2 treatment for another 24 h. Subsequently, cells were exposed to FC. (B) Atg7 silencing simulated the protective effect of E2 against the FC-induced cytotoxicity. (C) Preincubation with rapamycin significantly increased the levels of autophagy in each group except for FC-exposure group per se. The differentiated PC12 cells were pretreated with E2 followed by FC exposure. Five hours before FC exposure, the cells were preincubated with 25 nM rapamycin. (D) Preincubation with rapamycin significantly diminished the protective effect of E2 against FC-induced neurotoxicity. Data are expressed as means ± SD (n = 6), *p < 0.05, **p < 0.01.
Knockdown of Erα in E2-mediated suppression of FC-induced autophagy in PC12 cells
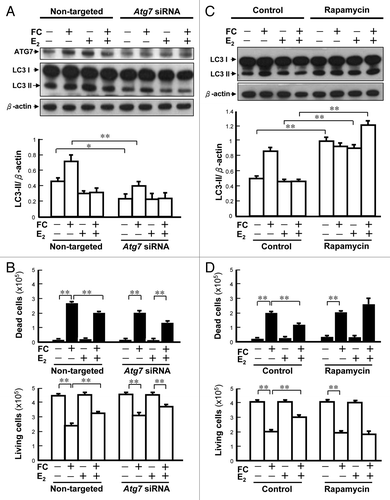
To examine whether ERα is essential for suppression of FC-induced autophagy, the level of LC3-II was examined after knockdown Erα by siRNA. As shown in , the ERα protein level in the Erα siRNA-treated group was lower than that in the nontargeted siRNA-treated group. E2 suppressed the FC-induced autophagy in nontargeted siRNA-treated group by 53 ± 8.2% (p < 0.01). The suppression of FC-induced LC3-II by E2 was diminished by Erα siRNA pretreatment (). The LC3 immunocytochemistry showed a similar trend to the western blot analysis (). In addition, the protective effect of E2 on FC-induced cytotoxicity was also diminished (p < 0.01) by Erα siRNA ().
Figure 3. Involvement of ERα in the E2-mediated suppression of FC-induced autophagy in PC12 cells. (A) Erα RNA silencing decreased the levels of ERα. The differentiated PC12 cells were pre-treated with Erα siRNA (50 nM) for 24 h, followed by E2 treatment for an additional 24 h before FC exposure. (B) Erα RNA silencing diminished the suppression of FC-induced LC3 lipidation by E2. (C) Erα RNA silencing diminished the suppression of LC3 immunoreactivity by E2. In the nontargeted siRNA treated group, E2 suppressed the FC-induced LC3 immunoreactivity. (D) Erα silencing diminished the protective effect of E2 against FC-induced neurotoxicity. Data are expressed as means ± SD (n = 6), *p < 0.05, **p < 0.01.
Sex difference in FC-induced autophagy and autophagic cell death in the rat striatum
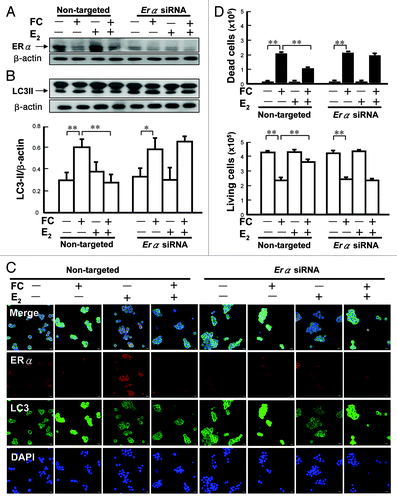
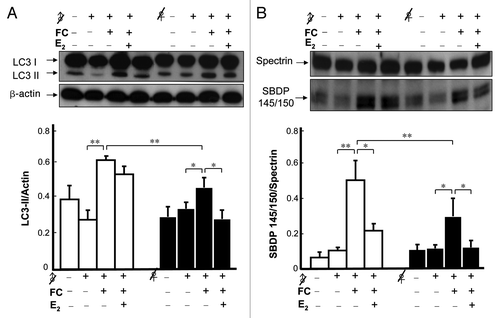
To examine the sex difference in FC-induced autophagy, brain injury, behavioral deficit, the levels of LC3 aggregation and LC3-II protein abundance in the CN of both male and female rats were compared. Autophagic cell death was identified by TUNEL(+) BECN1 immunoreactive cells with intact (round) nuclei (). The results demonstrated that the levels of FC-induced LC3 aggregation, TUNEL(+) BECN1 immunoreactive cells, and lipidated LC3-II were lower in the CN of female rats than they were in males (). In addition, the level of α-II spectrin breakdown products (SBDP 145/150) was lower in female rats than it was in males after FC infusion (). Moreover, both the FC-induced lesion ratio () and forelimb use asymmetry ratio () were lower in female rats than it were in males.
Figure 4. Sex differences in FC-induced autophagic signaling in the rat striatum. (A) FC increases more LC3 aggregation around the neuronal nucleus in male than in females. LC3 antibody followed by a secondary antibody conjugated with FITC and NeuN were used to stain LC3 aggregation (green) and neuronal nuclei (red), respectively. Nuclei were stained by DAPI. Arrows indicate LC3 aggregation around the NeuN positive nucleus. (B) FC-induces more autophagic cell death in male than in female rats. Rat striatum was stained using TUNEL and BECN1 for DNA damage (green) and autophagy (red), respectively. The merged views show round nuclei (yellow) depicting TUNEL(+) BECN1 immunoreactive cells with an intact nucleus, which is referred to as autophagic cell death and is indicated by arrows. (C) The basal level and level of FC-induced autophagy were lower in the CN of female than in male rats. (D) The level of FC-induced cleavage of spectrin was lower in the CN of female rats than it was in males. The levels of SBDP 145/150 in the CN of male and female rats with or without infusion of FC are shown as a ratio of SBDP 145/150/spectrin acting as an index of severity of injury. (E) The lesion ratio of FC-induced brain injury was lower in the CN of female rats than it was in males. The hemispheric area of the CN was quantified according to the density of the hematoxylin and eosin-stained tissue section by Image-pro plus software. The lesion ratio was estimated by dividing the hemispheric volume of the CN on the ipsilateral side by that on the contralateral side. (F) FC induces more severe behavioral deficit in male than in female rats. Forelimb use asymmetry ratio depicting an index of behavioral deficit. Data are expressed as means ± SD (n = 6), *p < 0.05, **p < 0.01.
Role of E2 in the sex difference in FC-induced autophagy and injury in the rat striatum
Our previous result showed that E2 plays a critical role in mediating the sex dimorphism in FC-induced neurological deficit and brain lesion examined by histology using fixed samples.Citation7 In the present study, to get better correlation between level of autophagy and injury severity, the level of lipidated LC3 and the ratio of SBDP 145/150/spectrin (instead of lesion ratio) were analyzed simultaneously using the same tissue samples in each groups. As shown in , intrastriatal infusion of FC induced a 33 ± 3.2% (p < 0.05) higher level of LC3-II in the CN of male rats than in females. The result of striatal injury showed that the level of SBDP 145/150 in FC-infused female rats was 40 ± 11.5% (p < 0.01) lower than that in males. Subcutaneous implantation of E2 24 h prior to infusion of FC decreased the levels of the FC-induced autophagy in castrated females by 39 ± 9.6% (p < 0.05) but only slightly decreased it in castrated males. Furthermore, E2 implantation decreased by 46 ± 6.4% (p < 0.05) and 42.9 ± 8.3% (p < 0.05) the FC-induced cleavage of α-II spectrin in female and male castrated rats in vivo, respectively ().
Figure 5. Effect of E2 on FC-induced autophagy and injury in the CN of castrated rats. (A) The level of FC-induced autophagy was lower in the CN of ovariectomized females (♀ with a slash) than it was in castrated males (♂ with a slash), and E2 implantation significantly decreased the levels of FC-induced autophagy in ovariectomized females. (B) The severity of FC-induced injury was lower in the CN of female rats than it was in males and E2 implantation significantly decreased the ratios of SBDP 145/150/spectrin in both female and male castrated rats. Data are expressed as means ± SD (n = 6), *p < 0.05, **p < 0.01.
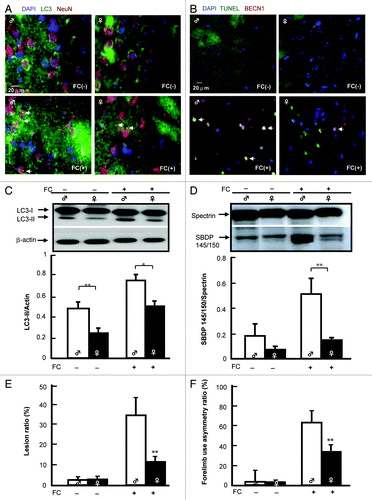
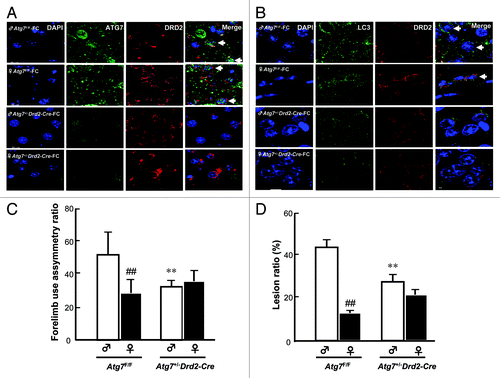
Effect of Atg7 conditional knockout in DRD2 neurons on the sex difference in FC-induced injury in the CN
To confirm the participation of autophagy in the sex difference in FC-induced injury, the behavior deficit and lesion ratio in CN of Atg7+/–Drd2-Cre mice (heterozygote conditional knockout Atg7 in DRD2 neurons) were compared with those in wild-type Atg7F/F mice. The Atg7 knockout efficiency in DRD2 neurons was checked after immunohistochemical staining of both ATG7 and LC3 in DRD2 neurons. As shown in , in Atg7 F/F mice, ATG7 immunoreactivity was observed in DRD2 neurons in CN, while, in CN of Atg7+/–Drd2-Cre mice, ATG7 immunoreactivity in DRD2 neurons was diminished. The level of LC3 aggregation was higher in the CN of Atg7F/F male mice than in females and conditional knockout of Atg7 significantly decreased the level of LC3 aggregation in both male and female mice (). Moreover, both the behavioral deficit and lesion ratio showed a significant sex difference in wild-type Atg7F/F mice, whereas no sex difference in either behavioral deficit or lesion ratio was observed in Atg7+/–Drd2-Cre mice. Moreover, in the male group, both the FC-induced behavioral deficit and lesion ratio in the CN of Atg7+/–Drd2-Cre mice were significantly lower than those in Atg7F/F mice (, respectively).
Figure 6.Atg7 conditional knockout in DRD2 neurons abolished the sex difference in FC-induced CN injury. Knockout of Atg7 decreased the ATG7 immunoreactivity (A) and LC3 aggregation (B) in DRD2 neurons in both male and female mice. The brain tissues containing the CN from male or female mice with (Atg7+/–D rd2-Cre) or without (Atg7F/F) Atg7 conditional knockout were sampled 2 d after FC infusion and sectioned at 10 μm thickness. ATG7 antibody or LC3 antibody followed by a secondary antibody conjugated to FITC and DRD2 antibody followed by a second antibody conjugated to rhodamine were used to stain for ATG7 or LC3 (green) and DRD2 neurons (red), respectively. Simultaneously, nuclei were stained with DAPI. The nuclei surrounded by cytosolic ATG7 or LC3 in DRD2 neuron depict ATG7-containing or autophagic DRD2 neurons as indicated using arrows. (C) Knockout of Atg7 significantly decreased the FC-induced behavioral deficit in male mice and diminished the sex difference in FC-induced behavioral deficit. Forelimb use asymmetry ratio depicting an index of behavioral deficit. (D) Knockout of Atg7 significantly decreased the level of FC-induced striatal injury in male mice and diminished the sex difference in FC-induced striatal lesion ratio. Data are expressed as means ± SD (n = 6). *p < 0.05, **p < 0.01 compared with the sham control sex-matched group. ##p < 0.01 compared with the male sham.
Discussion
The present study yielded several interesting findings. First, the protective effect of E2 on FC-induced neurotoxicity in PC12 cells was simulated by silencing of Atg7, and was diminished by rapamycin preincubation. Second, knockdown of Erα diminished the suppression of FC-induced autophagy by E2. Third, levels of autophagy and injury in the CN were lower in female rats than in males after FC infusion. Fourth, E2 replacement significantly decreased levels of FC-induced autophagy in ovariectomized rats. Finally, conditional knockout of Atg7 in DRD2 neurons diminished the sex difference of both FC-induced lesion ratio and behavioral deficit in the CN. These results suggest that suppression of FC-induced autophagy through an ERα dependent pathway might participate in the E2-mediated neuroprotection on FC-induced brain injury in female rats. To our knowledge, this is the first demonstration that autophagy participates in the E2-mediated neuroprotection against iron-induced brain injury. This finding opens up the prospect for a therapeutic strategy targeting autophagic inhibition for patients suffering from ICH or neurodegeneration caused by iron overload.
Autophagy is essential for maintaining homeostasis and cell survival. However, autophagic cell death has been recognized in conditions of oxidative stress.Citation15 A previous report indicated that treatment with rapamycin potentiated oxidative stress-induced cell death.Citation16 Another report indicated that inhibition of autophagy switched the mode of the cell death from apoptosis to necrosis in neonatal hypoxia-ischemia-induced brain injury.Citation17 Therefore, whether autophagy plays a beneficial or harmful role in different kinds of brain injury remains controversial. A recent report indicated that iron-induced autophagy may contribute to the brain injury after ICH.Citation9 However, no direct evidence has been provided yet. The present study demonstrated that most of the TUNEL(+) nuclei in PC12 neurons were surrounded by a high level of cytosolic BECN1 immunoreactivity after FC exposure (). Preincubation with rapamycin enhanced the neurotoxicity of FC (). The in vivo result of FC-induced CN injury also showed that most of the nuclei of the TUNEL(+) cells expressing BECN1 were round (), which is observed in autophagic cell death.Citation18 In addition, the higher level of FC-induced autophagy () is associated with greater severity of brain injury in male rats than in females (). Moreover, in vivo conditional knockout of Atg7 in DRD2 neurons significantly decreased the FC-induced striatal injury in males (). Taken together, these data suggest that autophagic cell death may contribute to the brain injury caused by iron overload, particularly in males.
“Stroke has been recognized as a sexually dimorphic disease.Citation19 Premenopausal women have a better survival rate than men after ICH.Citation3,Citation4 Animal study showed that brain edema was significantly severe in female as compared with that in male rats.Citation20 Hormones (e.g., E2) and sex have been shown to affect outcome after ICH in various animal models of ICH.Citation6,Citation20,Citation21 In the FC-infused animal model, the severity of the brain injury in males was higher than that in female rats and E2 treatment reduced the severity of brain edema in male ratsCitation21 and decreased the lesion ratio of brain injury and the behavioral deficit in both male and female castrated rats.Citation7 These results imply that there are sex differences in the mechanisms whereby neurons respond to injury and E2 may play a role in sex dimorphism of brain injury after ICH. However, previous in vitro studies have shown that neurons derived from male rat are more sensitive to excitotoxicity than neurons derived from female rat.Citation22 These in vitro studies implicate that there is an innate sexual dimorphism independent of circulating sex steroids. The present in vitro study, using PC12 cells but not neurons from male and female rats, showed that E2 significantly decreased the ratio of SBDP 145/150/spectrin () and increased the number of living cells after FC exposure (). The in vivo study showed that the severity of FC-induced injury was lower in the CN of female rats than males and E2 implantation significantly decreased the ratio of SBDP 145/150/spectrin in castrated male to the level of castrated female rats (). Although the innate sex dimorphism can not be excluded based on the current results, E2 may play a critical role in the sex dimorphism of FC-induced brain injury.”
Sex difference in neuronal autophagy has been first reported by Du L et al., who demonstrated that neurons from males more readily undergo autophagy and die during starvation, whereas neurons from females survive longer.Citation23 The result of the present in vitro study showed that E2 decreases the level of FC-induced autophagy. In addition, the protection of E2 against FC-induced neurotoxicity is simulated by Atg7 siRNA preincubation and is eliminated by rapamycin preincubation (). The present results further show that the level of FC-induced autophagy is lower in female rats than in males and E2 replacement significantly suppresses the FC-induced autophagy in ovariectomized females () suggesting that the suppression of FC-induced autophagy by E2 contributes to the less severity of brain injury in females. These data further imply that autophagy might be another factor involved in the sexual dimorphism of FC-induced brain injury. Greater therapeutic effort should be emphasized in male patients to suppress the autophagic cell death caused by iron overload.
It is well known that E2 exerts protective actions against various forms of neuronal injury including iron-induced brain injury in rats.Citation7 Multiple pathways may transmit the neuroprotective effect of E2.Citation24 However, the mechanism underlying how E2 modulates autophagy remains unknown. Previous findings have shown that levels of both mRNA and protein of ERα, but not ERβ, in the CN are higher in female rats than in males.Citation7 The present result showed that E2 implantation significantly decreased the ratio of SBDP 145/150/spectrin in both male and female group () implicating an ERα-independent protective mechanism of E2 in males. While E2 replacement significantly inhibited the FC-induced autophagy in females, but not in males () suggesting a sex dimorphic effect of E2 on autophagy. Furthermore, knockdown of ERα diminished the E2-mediated suppression of FC-induced autophagy in PC12 cells (). These results support the hypothesis that E2 suppresses FC-induced autophagy via an ERα-dependent pathway. However, further investigation is needed to explore how E2/ERα suppresses FC-induced autophagy.
In conclusion, suppression of FC-induced autophagy by E2 via ERα contributes to the less brain injury caused by iron overload in female rats than in males. Our results provide important information for developing sex-specific therapeutic strategies to prevent brain dysfunction caused by hemorrhage and/or by neurodegenerative disease associated with iron overload.
Materials and Methods
PC12 cells
PC12 cells (4 × 105/well) were plated in a 6-well dish pre-coated with 0.1 mg/ml poly-d-lysine (Sigma-Aldrich, P1024) and grown in phenol red free RPMI medium (Gibco, 11835030) containing 5% heat-inactivated fetal bovine serum for 3 d. The active subunit of NGF (2.5S) (4 nM) (Invitrogen, 13257-019) was added to the cultured medium for two days to differentiate PC12 cells into neurons. The culture medium was then replaced by the medium containing 10 nmol/l E2 (Sigma-Aldrich, E2758). After E2 pretreatment for 24 h, cells were treated with FC (2.4 nmol/μl; Sigma-Aldrich) for 24 h before harvested.
Immunostaining and TUNEL staining
The paraffin-embedded brain tissues were cut into 10 μm serial sections. PC12 cells were harvested, fixed with 4% paraformaldehyde, and adhered on slides by cytocentrifugation. Rabbit anti-LC3B antibody (1:50; Sigma-Aldrich, L7543) and mouse anti-BECN1 antibody (1:50; BD Biosciences, 612112) were used for detections of LC3 aggregation and BECN1 immunoreactivity, respectively. NeuN antibody (1:100; Millipore Corporation, MAB377) was used to detect neuron. DAPI (Sigma-Aldrich) and TUNEL solution containing FITC-dUTP (In Situ Cell Death Detection Kit, Roche Diagnostics, 12 156 792 001) were used for nucleus labeling and detection of DNA fragmentation, respectively. Autophagic cell death was identified by the TUNEL(+) BECN1 immunoreactive positive staining cells with intact (round) nuclei.Citation25 Mouse monoclonal ERα antibody (1: 25, Leica Biosystems, 6008295) was used to detect ERα. FITC-conjugated AffiniPure goat anti-rabbit IgG (1:200, Jackson ImmunoResearch Laboratories, 111-095-003) and Rhodamine-conjugated AffiniPure goat anti-mouse IgG (1:50, Jackson ImmunoResearch Laboratories, 115-025-003) were used to recognize the primary antibodies. Images were acquired using a fluorescence microscope or an Olympus FV1000 confocal laser scanning microscope (Olympus, Fluoview FV1000).
Western blot analysis
An equal amount of protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a PVDF membrane, incubated with antibody against LC3 antibody (1:1000), α-II spectrin antibody (1:1000, Santa Cruz Laboratories, sc-7465), ERα (1: 25) or ATG7 antibody (1:1000, Biosensis, R-161-100) followed by a second antibody, and then visualized by ECL chemiluminescence (PerkinElmer, NEL 105). The amount of LC3-II (lipidated LC3) normalized by β-actin depeicts the level of autophagy.Citation26 The ratio of SBDP 145/150/spectrin acts as an index of severity of injury.
Atg7 and Erα RNA interference
The mixture of rat Atg7 siRNA contained four sequences: 3′-CAAAGUUAACAGUCGGUGUUU-5′, 5′-PACACCGACUGUUAACUUUGUU-3′; 3′-AGUGAAUGCCAGCGGGUUCUU-5′, 5′-PGAACCCGUGGCAUUCACUUU-3′; 3′-GUGGAGGAACUCAUCGAUAUU-5′, 5′-PUAUCGAUGAGUUCCUCCAGUU-3′; and 3′-CCCAGAAGAAGUUGAAGGAUU-5′, 5′-PUCGUUCAACUUCUUCUGGGUU-3′ (Dharmacon, NM_001012097). The sequences of Erα siRNA were 3′-AGUUGAUCAUAUGAACCAGCU-5′, 5′-CUGGUUCAUAUGAUCAACUGG-3′. A 5 μl (20 μmol/l) Atg7 siRNA or a 5 μl (10 μmol/l) Erα siRNA was mixed with 5 μl transfection reagent for 15 min, and then diluted with 990 μl OPTI-MEM medium. Dharmacon siCONTROL non-targeted siRNA (D-001210-01-05) was used as a negative control.
Enhancement of autophagy by rapamycin preincubation
To enhance autophagic machinery, 5 h before FC exposure, the PC12 cells were preincubated with the equivalent of 25 nM rapamycin (LC Laboratories, R-5000), which was encapsulated in a polycaprolactone-grafted chondroitin sulfate amphiphilic polymer by dialysis to improve its water solubility.
Animals
Eighty-four 12-week-old male and female Sprague Dawley rats (350–420 g; LASC; Charles River Technology) were used. No animals were excluded after random grouping. Experimental procedures were approved by the Kaohsiung Medical University Committee for the Use of Experimental Animals. Castration was performed under anesthesia with pentobarbital (35 mg/kg body weight, intraperitoneally) 2 weeks before E2 implantation. A Silastic tube (2 mm inner diameter, 30 mm in length) containing 0.38 mmol/l E2 (Sigma-Aldrich, E8515) was implanted subcutaneously 24 h before FC infusion. Ten microliters of FC (1 nmol/μl) was infused into the right CN (coordinates: 0.2 mm anterior, 3.5 mm lateral, and 5.5 mm ventral to bregma) at a rate of 10 μl/min using a microinfusion pump (VC50; CMA Microdialysis). Ten microliters of saline was infused in the sham control.
Forelimb use asymmetry test
Each individual rat was placed in a transparent cylinder (25 cm in diameter and 30 cm in height) in the dark, and the use of ipsilateral limbs (I), contralateral limbs (C), or simultaneous use of both forelimbs (B) was observed for a 5 min period. The test was randomized, blinded, and repeated twice in each rat. The forelimb use asymmetry ratio was calculated using the following equation: [I/(I + C + B)] − [C/(I + C + B)].Citation27
Histological examination of the brain injury
After hematoxylin and eosin (H&E) staining, the brain lesion in every fifteenth section of the CN was analyzed using Image-pro Plus software (Universal Imaging Corp.) to determine the staining intensity of the CN area. The total hemispheric volume was integrated from the hemispheric areas of 20 sections. The lesion ratio was estimated by dividing the hemispheric volume of the CN on the ipsilateral side by that on the contralateral side.
Conditional knockout of Atg7 in DRD2 neurons
The Atg7-conditional knockout mice (Atg7 flox/flox; Atg7F/F) (a gift from Dr. Masaaki Komatsu, Laboratory of Frontier Science, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan.) were cross-bred with transgenic mice expressing Cre recombinase under the control of a Drd2 promoter (Drd2-Cre) (purchased from Mutant Mouse Regional Resource Centers) to produce mice deficient for Atg7 specifically in the DRD2-containing neurons, which is abundant in CN. The F1 heterozygote littermates (Atg7+/–Drd2-Cre) were used in this study. Atg7 F/F male and female mice were used as control groups. Three microliters of FC (1 nmol/μl) was infused into the right CN (coordinates: 0.2 mm anterior, 2.5 mm lateral, and 3.5 mm ventral to bregma) at a rate of 2 μl/min. Two days after FC infusion, the brain tissue containing CN was sampled for the evaluation of histological lesion after behavioral functional test. The knockout efficiency of Atg7 in DRD2 neurons was checked by immunohistochemical staining.
Statistical analysis
FC-induced injury was compared between brains from males and females using a two-way ANOVA followed by a Scheffé post hoc test. Data for the effects of E2, Atg7 siRNA, rapamycin, or Erα siRNA on FC-cytotoxicity or autophagy were analyzed using a multi-way ANOVA to determine the effect of each factor and the interaction between two factors. Significance was accepted at p < 0.05.
| Abbreviations: | ||
| Atg7 | = | gene of autophagy-related protein 7 |
| BECN1 | = | Beclin 1 |
| CN | = | caudate nucleus |
| DRD2 | = | dopamine receptor 2 |
| ERα | = | estrogen receptor α |
| FC | = | ferrous citrate |
| ICH | = | intracerebral hemorrhage |
| LC3 | = | microtubule-associated protein 1 light chain 3 |
| SBDP 145/150 | = | spectrin breakdown products with molecular weight of 145 or 150 |
Acknowledgment
This work was supported by research grants from the National Science Council of Taiwan (99-2320-B-037-024-MY3 and 99-2314-B-037-025 to C.H.; 99-2314-B-037-025 to C.-L.L.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke 2007; 38:Suppl 759 - 62; http://dx.doi.org/10.1161/01.STR.0000247868.97078.10; PMID: 17261733
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006; 5:53 - 63; http://dx.doi.org/10.1016/S1474-4422(05)70283-0; PMID: 16361023
- Zia E, Engström G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke 2009; 40:3567 - 73; http://dx.doi.org/10.1161/STROKEAHA.109.556324; PMID: 19729603
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9:167 - 76; http://dx.doi.org/10.1016/S1474-4422(09)70340-0; PMID: 20056489
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab 2004; 24:487 - 94; http://dx.doi.org/10.1097/00004647-200405000-00002; PMID: 15129180
- Auriat A, Plahta WC, McGie SC, Yan R, Colbourne F. 17beta-Estradiol pretreatment reduces bleeding and brain injury after intracerebral hemorrhagic stroke in male rats. J Cereb Blood Flow Metab 2005; 25:247 - 56; http://dx.doi.org/10.1038/sj.jcbfm.9600026; PMID: 15678126
- Chen TY, Tsai KL, Lee TY, Chiueh CC, Lee WS, Hsu C. Sex-specific role of thioredoxin in neuroprotection against iron-induced brain injury conferred by estradiol. Stroke 2010; 41:160 - 5; http://dx.doi.org/10.1161/STROKEAHA.109.562850; PMID: 19940280
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun 2004; 313:453 - 8; http://dx.doi.org/10.1016/j.bbrc.2003.07.023; PMID: 14684184
- He Y, Wan S, Hua Y, Keep RF, Xi G. Autophagy after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab 2008; 28:897 - 905; http://dx.doi.org/10.1038/sj.jcbfm.9600578; PMID: 17987045
- Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: a protein kinase switching between life and death. Pharmacol Res 2004; 50:545 - 9; http://dx.doi.org/10.1016/j.phrs.2004.03.007; PMID: 15501691
- Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci 2009; 29:13823 - 36; http://dx.doi.org/10.1523/JNEUROSCI.3574-09.2009; PMID: 19889994
- Yu J, Henske EP. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res 2006; 66:9461 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-06-1895; PMID: 17018601
- Weiss ES, Wang KK, Allen JG, Blue ME, Nwakanma LU, Liu MC, et al. Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest. Ann Thorac Surg 2009; 88:543 - 50; http://dx.doi.org/10.1016/j.athoracsur.2009.04.016; PMID: 19632410
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 1998; 78:189 - 225; PMID: 9457173
- Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 2008; 172:454 - 69; http://dx.doi.org/10.2353/ajpath.2008.070876; PMID: 18187572
- Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem 2010; 112:366 - 76; http://dx.doi.org/10.1111/j.1471-4159.2009.06463.x; PMID: 19878437
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 2008; 32:329 - 39; http://dx.doi.org/10.1016/j.nbd.2008.07.022; PMID: 18760364
- Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis 2009; 33:143 - 8; http://dx.doi.org/10.1016/j.nbd.2008.09.009; PMID: 18948195
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma 2000; 17:367 - 88; http://dx.doi.org/10.1089/neu.2000.17.367; PMID: 10833057
- Nakamura T, Hua Y, Keep RF, Park JW, Xi G, Hoff JT. Estrogen therapy for experimental intracerebral hemorrhage in rats. J Neurosurg 2005; 103:97 - 103; http://dx.doi.org/10.3171/jns.2005.103.1.0097; PMID: 16121980
- Gu Y, Xi G, Liu W, Keep RF, Hua Y. Estrogen reduces iron-mediated brain edema and neuronal death. Acta Neurochir Suppl 2010; 106:159 - 62; http://dx.doi.org/10.1007/978-3-211-98811-4_29; PMID: 19812941
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 2004; 279:38563 - 70; http://dx.doi.org/10.1074/jbc.M405461200; PMID: 15234982
- Du L, Hickey RW, Bayir H, Watkins SC, Tyurin VA, Guo F, et al. Starving neurons show sex difference in autophagy. J Biol Chem 2009; 284:2383 - 96; http://dx.doi.org/10.1074/jbc.M804396200; PMID: 19036730
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol 2001; 63:29 - 60; http://dx.doi.org/10.1016/S0301-0082(00)00025-3; PMID: 11040417
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 2004; 304:1500 - 2; http://dx.doi.org/10.1126/science.1096645; PMID: 15131264
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 2007; 3:542 - 5; PMID: 17611390
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke 2002; 33:2478 - 84; http://dx.doi.org/10.1161/01.STR.0000032302.91894.0F; PMID: 12364741