Abstract
Mantle cell lymphoma (MCL) is an aggressive neoplasm, which lacks effective therapy. The mechanistic target of rapamycin (MTOR) kinase inhibitor everolimus (RAD001) has shown activity in preclinical and clinical models of MCL, despite the fact that its mechanism of action has not been fully elucidated. We found that everolimus activity in MCL cells is closely linked to AKT phosphorylation status, and that the prevention of AKT rephosphorylation upon everolimus treatment by means of a selective AKT inhibitor, greatly enhances everolimus activity. Furthermore, our data show that an accumulation of autophagic vacuoles correlates with a lack of efficacy of dual AKT-MTOR targeting and that the complete therapeutic potential of this strategy can be restored by ATG gene selective knockdown or secondary inhibition of autolysosome formation by hydroxychloroquine. We thus demonstrated for the first time that the use of an autophagy inhibitor can overcome resistance to the combination of MTOR and AKT inhibitors in MCL cell lines and primary samples, demonstrating the prosurvival role of autophagy in AKT-MTOR compromised cells, and pointing out some potential opportunities using this triple combinational strategy in hematological malignancies.
The precise role that autophagy plays in cancer development, disease progression, and the response to anticancer therapies, is controversial. At one level, autophagy has been suggested to function as a tumor suppressor pathway that prevents cancer development, because it prevents the proliferation, and/or induces the death, of cells bearing mutations. Furthermore, autophagy can also provide protection from cell death in cells exposed to different stress inducers and common chemotherapeutics in several cancer models. Therefore, the impact of autophagy induction on the efficacy of antitumoral agents can be highly variable and cell type- and treatment-dependent. This evolutionarily conserved role of autophagy has stimulated research to determine whether the modulation of this phenomenon may interfere with cancer cell response to cytotoxic therapy. Most efforts have focused on using autophagy inhibitors in tumor cell lines with high levels of basal autophagy or in conjunction with agents that directly stimulate autophagy signaling pathways such as MTOR inhibitors or dual PI3K-MTOR inhibitors.
Among the cancer models that may directly benefit from targeting the constitutively activated PI3K-AKT-MTOR signaling pathway, are the majority of B-cell neoplasms, and especially mantle cell lymphoma. The ability of various rapalogs to counteract MTOR activity by targeting MTORC1 complexes has been evaluated in both clinical and preclinical studies in MCL, showing a moderate success as monotherapy with mild toxic effects, and granting the approval of temsirolimus (CCI-779) by the European Medicines Agency (EMEA) for the treatment of relapsed and refractory MCL patients. However, in vitro studies have suggested that the effectiveness of these agents may be stifled in part by strong MTORC1-dependent negative feedback loops that become inactive upon MTORC1 inhibition, paradoxically leading to survival-promoting events like the activation of AKT. Among the possible mechanisms related to resistance to MTORC1 inhibition, activation of autophagic processing has also been observed some years ago in MCL cells exposed to temsirolimus.
In our recent study, aimed to characterize the molecular bases of sensitivity/resistance to everolimus (RAD001) in MCL, we showed that the development of cell resistance to dual targeting of AKT and MTOR by means of an isoselective AKT inhibitor and everolimus, respectively, relies on the degree of autophagy induction. Indeed, while everolimus induces a tumor-selective, dose-dependent cytotoxicity in the majority of MCL cases, related to G1 cell cycle arrest and rapid downregulation of the MTOR downstream targets phospho-RPS6 and phospho-EIF4EBP1, a rephosphorylation of AKT at Ser473 presumably linked to the activation of an MTORC1-dependent feedback loop, may limit the efficacy of everolimus. The full activity of the rapalog requires its combination with an isoselective AKT inhibitor (AKTi-1/2) for complete AKT-MTOR axis inhibition and synergistic antitumoral activity. However, we observed that those MCL samples that are weak responders to everolimus as a single agent still harbor a high viability rate despite the complete AKT-MTOR axis inhibition, in accord with a previous observation in follicular lymphoma. As accumulating evidence suggests that autophagy is one of the major processes functionally involved in cancer cell survival after AKT and MTOR inhibitor exposure, we performed the quantification of autophagy by western blot, flow cytometry and immunofluoresence assays, and found an increase in LC3B-II levels and the accumulation of autolysosomes in cells resistant to this combination therapy. We further demonstrated that autophagy controls the MCL response to MTOR-AKT inhibitors, as the triple knockdown of ATG7, ATG5 and ATG3, allows MCL cells to undergo apoptosis upon exposure to the combination.
In the clinic, the blockers of autophagosome-lysosome fusion hydroxychloroquine and chloroquine are being tested in a number of trials in patients with distinct hematological malignancies, such as multiple myeloma (in combination with the proteasome inhibitor bortezomib) or in chronic myeloid leukemia (in combination with the HDAC inhibitor vorinostat), but no data on their efficacy and safety in aggressive lymphomas like MCL are available. We tested this inhibitor for the first time in MCL primary cultures and we found that the addition of hydroxychloroquine to everolimus-AKTi-1/2 treatment allows the complete processing of LC3B and the full activation of the intrinsic apoptotic program in MCL cells, as demonstrated by mitochondrial depolarization, ROS production, phosphatidylserine exposure and CASP3-CASP7 activity ().
Figure 1. AKT-MTOR and autophagy triple targeting as an effective antitumoral therapy in MCL. Treatment with AKTi-1/2, an isoselective AKT inhibitor, prevents AKT rephosphorylation upon everolimus-mediated inhibition of MTORC1, thus enhancing the activity of the rapalog in MCL cells. However, the degree of autophagy induced by dual MTOR-AKT inhibition determines the fate of the cells, which can undergo either apoptotic cell death or survival. In low-responsive everolimus-AKTi-1/2 MCL cells, pro-survival autophagy can be counteracted by autophagy inhibitors such as bafilomycin A1 (Baf. A1) or hydroxychloroquine (HCQ), thus restoring the cytotoxic potential of MTOR-AKT targeting.
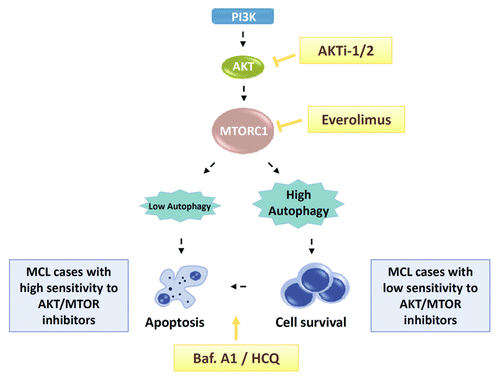
Thus, these results provide the proof-of-principle and rationale for further clinical evaluation of AKT-MTOR and autophagy triple targeting in MCL patients, and offer a glimpse of the potential opportunities that this combinational strategy might hold for the treatment of B-cell neoplasms.
Acknowledgments
This work was supported by Fondo de Investigacíon Sanitaria (PI09/0060; to G.R.), Ministerio de Ciencia e Innovacíon (SAF 09/9503; to D.C.), Redes Temáticas de Investigacíon Cooperativa de Cáncer from the Instituto de Salud Carlos III (RED 2006-20-014 to D.C.) and Generalitat de Catalunya (2009SGR967 to D.C.). L.R. is recipient of a predoctoral fellowship from IDIBAPS. This work was performed, in part, at the Esther Koplowitz Center, Barcelona.