Abstract
Stimulation of apoptosis has been reported as the primary mechanism of tumor cell death induced by alpha-tocopheryloxyacetic acid (α-TEA), an esterase-resistant, semi-synthetic derivative of vitamin E (R-R-R-α-tocopherol). New information now shows that α-TEA also triggers tumor cell autophagy and promotes antigen cross-presentation. Autophagosome-enriched fractions of α-TEA-treated tumor cells (α-TAGS) efficiently cross-primed antigen-specific CD8+ T cells and vaccination with dendritic cells (DC) pulsed with α-TAGS reduced lung metastases and increased survival of tumor-bearing mice. Taken together, these observations suggest that both autophagy and apoptosis signaling programs are activated in tumor cells by α-TEA treatment and may contribute to tumor cell death. We propose that autophagy-dependent enhancement of cross-presentation is a novel mechanism of α-TEA-mediated tumor immunity and that α-TEA can be exploited as an adjuvant to enhance the antitumor response.
α-TEA-Induced Autophagy Precedes Apoptosis and Decreases Tumor Cell Survival
Alpha-tocopheryloxyacetic acid is a stable, esterase-resistant semi-synthetic derivative of naturally occurring vitamin E. It is derived from vitamin E by replacing the hydroxyl group at the number 6 carbon of the phenolic ring of the chroman head with an acetic acid residue linked by an ether bond. Studies on the mechanism of α-TEA antitumor activity have implicated apoptosis as a primary mode of α-TEA-induced tumor cell death, a process that involves reactive oxygen species (ROS) production, mitochondrial depolarization and activation of the intrinsic apoptotic program that ultimately results in tumor cell destruction. Our recent study is the first to demonstrate that α-TEA stimulates tumor cell autophagy in addition to its well-described pro-apoptotic function. We found that α-TEA stimulated tumor cell autophagy in parallel with apoptosis and that autophagy preceded apoptosis by a few hours. Interestingly, pretreatment of GFP-LC3-expressing tumor cells with the pan-caspase inhibitor, zVAD-fmk, increased LC3 puncta formation by α-TEA while concomitantly inhibiting apoptosis as expected. Notably, unlike other reports where apoptosis induction during stressful conditions results in cleavage of BECN1, we only observed a modest decrease in BECN1 protein levels without any discernable evidence of BECN1 cleavage, suggesting that BECN1 cleavage may not be the critical regulatory node during α-TEA-induced apoptosis/autophagy cross-talk. We also observed that doxycycline-induced shRNA knockdown of the autophagy pathway gene Atg12 increases tumor cell survival in a long-term colony formation assay suggesting that α-TEA-induced autophagy can eventually result in tumor cell death.
α-TEA Enriched Autophagosome Fraction (α-TAGS) Stimulates Antigen Cross-Presentation and Enhances in Vivo Antitumor Activity
Previously, we reported that α-TEA-treatment of tumor-bearing mice enhances the antitumor immune response by causing changes in the tumor microenvironment, and that the therapeutic efficacy of orally active α-TEA is dependent on an intact T cell compartment. The knowledge that during autophagy, cellular components including viral or endogenous tumor-associated antigens become available for cross-presentation by professional antigen-presenting cells (APC) to prime antigen- or tumor-specific T cell responses, led us to investigate if autophagy induction by α-TEA promoted antigen cross-presentation. In our recent study, we report for the first time that α-TAGS generated by treating ovalbumin (OVA)-gene transduced tumor cells in vitro with α-TEA, is an efficient antigen carrier. Dendritic cells pulsed with OVA-containing α-TAGS (OVA-α-TAGS) efficiently stimulate antigen cross-presentation and enhance proliferation of OVA-specific OT-1 CD8+ T cells in vitro. Pharmacological as well as genetic inhibition of the autophagy pathway reduces T cell proliferation and interferon-gamma (IFNγ) secretion by OVA-α-TAGS-stimulated OT-1 CD8+ T cells, demonstrating that autophagy is essential for efficient antigen cross-presentation and T cell activation. The improved antigen cross-presentation by α-TAGS-pulsed DC (α-TAGS-DC) observed in vitro, is also evident in vivo as intra-nodally injected α-TAGS derived from OVA-expressing tumor cells induce proliferation of adoptively transferred OT-I CD8+ T cells. More importantly, α-TAGS-DC vaccination of tumor-bearing mice reduces the incidence of experimental lung metastasis and prolonged survival of mice with established tumors. Taken together, these results suggest that tumor antigens in α-TAGS were cross-presented in vivo to stimulate an antitumor immune response.
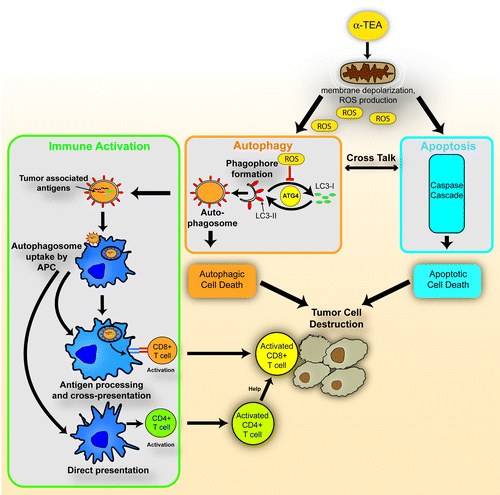
Based on these collective observations, we propose a model of the mechanism of α-TEA-mediated antitumor activity (). We have provided evidence that both autophagy and apoptosis signaling pathways are activated in tumor cells by α-TEA and that they contribute to α-TEA-mediated tumor cell destruction (, orange and blue pathways). Autophagy induction by α-TEA may be triggered by reactive oxygen species (ROS) production in tumor cell mitochondria (). LC3-II generation is regulated by the autophagy pathway gene product ATG4. ATG4 cleaves the C terminus of cytosolic LC3 to reveal a glycine residue (LC3-I) for attachment of phosphatidylethanolamine (PE) to form lipidated LC3-II on the cell membrane of the maturing phagophore. ATG4 also acts as a deconjugating enzyme for recycling LC3-II back to the LC3-I form. ROS oxidizes the cysteine protease ATG4 (via H202) to prevent the delipidation of LC3, thus promoting stabilization of LC3-II and the generation of autophagosomes. We also propose that immune activation by α-TEA is dependent on a functional autophagy pathway (, green pathway). After antigen processing by APCs, tumor-associated antigens are cross-presented to CD8+ T cells or directly presented to CD4+ T cells, leading to the activation of these T cell populations. Activated CD8+ T effector cells, with or without CD4+ T cell help then contribute to the α-TEA-mediated tumor cell destruction ().
Figure 1. α-TEA-mediated antitumor activity is mediated by three mechanisms. First, α-TEA targets tumor cell mitochondria and generates reactive oxygen species (ROS), and causes membrane depolarization resulting in activation of the intrinsic apoptotic pathway (blue). Second, α-TEA triggers tumor cell autophagy and autophagic death (orange) that in addition to apoptotic cell death contributes to tumor cell destruction. Autophagosome formation requires lipidated LC3-II that is generated from LC3-I through attachment of a phosphatidylethanolamine (PE) residue. ATG4 catalyzes an earlier step needed for this PE conjugation, but also acts as a deconjugating enzyme for recycling LC3-I. Oxidation of ATG4 by ROS inhibits the delipidation of LC3, thus promoting accumulation of LC3-II and autophagosomes. Third, α-TEA also activates a tumor-specific immune response (green) by generation of autophagosomes containing tumor antigens that are taken up by professional antigen-presenting cells (APC). After processing, tumor antigens are either cross-presented to CD8+ T cells or directly presented to CD4+ T cells. The resulting antitumor T cell response is the third front of attack of the tumor leading to its destruction.
Due to its relative stability, lack of apparent toxicity in vivo as well as its ability to stimulate autophagy and enhance cross-priming of CD8+ T cells, α-TEA chemotherapy may have clinical relevance as an immunogenic adjuvant that could be combined with other immune modulators (e.g., anti-PDCD-1, anti-CTLA-4, anti-4-1BB ligand/TNFSF9, trastuzumab) to drive an effective, long-lasting antitumor immune response.
Acknowledgments
The study was supported by NIH grants (5RO1CA120552 to E.T.A., RO1CA10724375 and R21CA141278 to H.-M.H., and 2RO1CA111421 and 1RO1CA150925 to A.T.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.