Abstract
Impairment of autophagy in patients and animal models severely affects mechanically strained tissues such as skeletal muscle, heart, lung and kidney, leading for example to muscle dystrophy, cardiomyopathy and renal injury. However, the reason for this high reliance on autophagy remained largely elusive. Recent work in our lab now provides a possible explanation. We identified chaperone-assisted selective autophagy (CASA) as a tension-induced autophagy pathway essential for mechanotransduction in mammalian cells.
CASA represents a pathway for the selective degradation of chaperone clients in lysosomes. The client is initially recognized by a multichaperone complex comprised of the molecular chaperones HSPA8/Hsc70 and HSPB8/Hsp27, held together by the autophagy-inducing co-chaperone BAG3 (). The client becomes ubiquitinated by the HSPA8-associated ubiquitin ligase STUB1/CHIP, which cooperates with ubiquitin conjugation enzymes of the UBE2D family. Ubiquitination generates a degradation signal that is recognized by the autophagic ubiquitin adaptor SQSTM1/p62, leading to autophagosome formation and client degradation in lysosomes (). CASA is thus distinct from chaperone-mediated autophagy, which involves the translocation of chaperone clients displaying a KFERQ consensus-degradation motif directly across the lysosome membrane.
Figure 1. The CASA machinery integrates tension sensing, autophagosome formation and transcription regulation during mechanotransduction in mammalian cells. The machinery is localized at Z-disks in striated muscles and along actin stress fibers in smooth muscle and nonmuscle cells. A critical client of CASA is the actin-crosslinking protein FLN, which becomes unfolded and damaged under tension, leading to recognition and ubiquitination by the CASA complex. During FLN degradation autophagosome formation is triggered by the cooperation between BAG3, SYNPO2 and a VPS protein complex that facilitates the tethering and fusion of phagophores. BAG3 also uses its WW domain to contact LATS1/2 or AMOTL1/2, which are involved in the cytoplasmic sequestration of the transcription regulators YAP1 and WWTR1. BAG3 binding to LATS1/2 and AMOTL1/2 abrogates YAP1-WWTR1 sequestration and induces FLN transcription under tension. It remains to be elucidated whether BAG3 cooperates with HSPA8 and HSPB8 during transcription regulation. Ig, immunoglobulin-like; ECM, extracellular matrix.
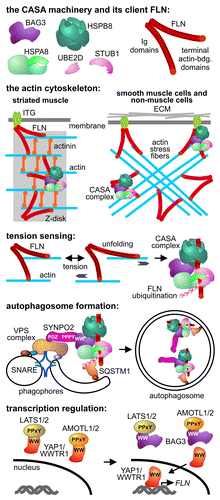
Intriguingly, CASA is essential for the adaptation of mammalian cells to mechanical strain. In striated skeletal muscles, the CASA machinery is concentrated at the Z-disk, a structure that mediates the anchoring and crosslinking of actin filaments (). Impairment of CASA leads to Z-disk disintegration in contracting muscles, causing muscle dystrophy and cardiomyopathy. In smooth muscle cells and nonmuscle cells the machinery localizes along actin stress fibers that form when tension is generated inside cells during adhesion and migration (). These processes are indeed severely impaired upon CASA inhibition, again illustrating the functional significance of the observed cytoskeleton association.
A critical client of CASA in mechanically strained cells is the cytoskeleton protein FLN/filamin, which crosslinks actin filaments at Z-disks and stress fibers (). FLN is a homodimer comprised of two ~250-kDa rods, each formed by an N-terminal actin-binding domain followed by 24 immunoglobulin-like (Ig) domains. Notably, FLN acts as a mechanosensor in mammalian cells. Externally applied force or intracellular tension triggers the disruption of Ig domain interactions and the unfolding of individual Ig domains, which affects the association of FLN with cytoskeleton components and signaling proteins. In addition, the resultant extension enables FLN to serve as a flexible linker between mechanically strained actin filaments (). The price for this flexibility, however, is the exposure of unfolded regions, prone to aggregation and irreversible damage. In this situation the CASA complex exerts vital protein quality control functions. We could show that BAG3, HSPA8 and HSPB8 bind in a cooperative manner to a mechanosensitive region of FLN, comprising Ig domains 19 through 21. Chaperone binding would reduce aggregate formation and promote refolding. However, if the folded state cannot be attained because of irreversible damage, cooperation between BAG3, STUB1 and SQSTM1 triggers FLN ubiquitination and autophagic disposal ().
Tension-induced unfolding of mechanosensors and cytoskeleton proteins apparently generates a situation comparable to heat stress. In both settings a depletion of the available pool of chaperones is the consequence. Strikingly, we observed that tension, just like heat stress, activates the heat shock transcription factor HSF1. This stimulates the compensatory expression of diverse chaperones and proteostasis factors, and also of BAG3, thereby increasing the capacity for autophagic disposal of unfolded proteins. The data unravel a chain of events for adjusting CASA activity to the extent of tension within the actin cytoskeleton. Indeed, BAG3 expression and CASA activity are strongly upregulated in contracting mouse muscles. In the current study, we induced different levels of tension in smooth muscle cells by stretching and by plating on surfaces of different stiffness. Furthermore, immune cells were analyzed in adhesion, spreading and migration assays, and were subjected to shear stress. In all cases, mechanical force triggered the expression of BAG3 and stimulated the activity of CASA. Our data demonstrate that CASA is a tension-induced autophagy pathway.
To gain more insights into CASA, we searched on a proteome-wide scale for human proteins that interact with the WW domain of BAG3. Such WW domains bind proline-rich motifs in partner proteins. Indeed, we could identify 72 novel putative BAG3 interactors, which preferentially contain PPPY or PPSY motifs. Among the identified interactors was synaptopodin 2 (SYNPO2, also known as myopodin), a Z-disk component and FLN binding partner. It turned out that SYNPO2 is an integral part of the CASA machinery and is essential for the formation of autophagosomes (). A PDZ domain at the N-terminus of SYNPO2 interacts with the vacuolar protein sorting 18 homolog (S. cerevisiae; VPS18). The latter protein is a core component of VPS complexes that recognize small GTPase RAB proteins on target membranes in preparation for membrane fusion. Although the RAB adaptors and RAB proteins, which are specifically involved in autophagosome formation, remain to be identified, SYNPO2 contacts ATG16L1- and LC3B-containing autophagosome precursor membranes (phagophores) through the associated VPS complex (). Moreover, we could detect the SNARE protein syntaxin 7, previously linked to phagophore fusion, in association with SYNPO2. The data reveal that SYNPO2 provides a link between the client-processing CASA chaperone complex and a membrane-tethering and fusion machinery that generates autophagosome membranes ().
BAG3 utilizes its WW domain not only to cooperate with SYNPO2 during autophagosome formation. Alternatively, PPxY-containing signaling proteins of the Hippo pathway, i.e., LATS1/2 and AMOTL1/2, are contacted by the BAG3 WW domain. The pathway controls organ development and tumorigenesis by modulating the activity of the transcription regulators YAP1 and WWTR1/TAZ. In mechanically strained cells, binding of BAG3 to LATS1/2 and AMOTL1/2 abrogates the cytoplasmic retention of YAP1 and WWTR1 and thereby induces the expression of proteins involved in cell adhesion, including FLN (). The BAG3 co-chaperone thus emerges as a dual function proteostasis factor. It facilitates the autophagic degradation of mechanically damaged cytoskeleton components and at the same time triggers a transcriptional response to compensate disposal. This dual function is essential for the homeostasis of mechanically strained tissues in mammals.
Acknowledgments
Work in the authors’ lab was supported by the Deutsche Forschungsgemeinschaft (SFB 635, SFB 645 and FOR 1352).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.