Abstract
Macroautophagy mediates recycling of intracellular material by a multistep pathway, ultimately leading to the fusion of closed double-membrane structures, called autophagosomes, with the lysosome. This event ensures the degradation of the autophagosome content by lysosomal proteases followed by the release of macromolecules by permeases and, thus, it accomplishes the purpose of macroautophagy (hereafter referred to as autophagy). Because fusion of unclosed autophagosomes (i.e., phagophores) with the lysosome would fail to degrade the autophagic cargo, this critical step has to be tightly controlled. Yet, until recently, little was known about the regulation of this event and the factors orchestrating it. A punctum in this issue highlights the recent paper by Noboru Mizushima and his collaborators that answered the question of how premature fusion of phagophores with the lysosome is prevented prior to completion of autophagosome closure.
Keywords: :
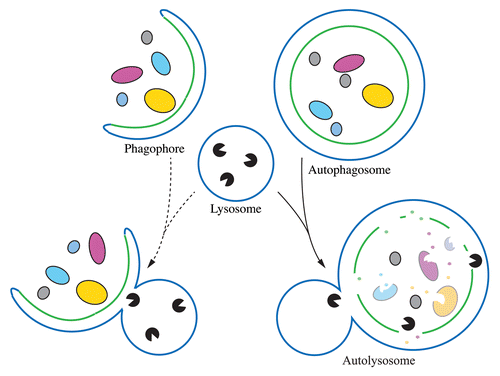
How fusion of the completed autophagosomes with the lysosome is achieved and regulated has remained elusive, in particular because autophagosomal proteins involved in this process were yet to be described. In their study, Itakura et al. identified the autophagosome-associated SNARE protein Syntaxin17 (STX17), and demonstrated its function in mediating the fusion of autophagosomes with the lysosome/endosome.Citation1 Interestingly, STX17 does not colocalize with the phagophore; instead, it is recruited specifically to completed autophagosomes. This finding revealed the unpredicted and very elegant model in which the late recruitment of STX17 to the autophagosome is a key factor in preventing premature fusion with the lysosome. Thus, autophagic degradation cannot start until the autophagosome cargo is completely enclosed, which ensures that the cargo is delivered into the lysosome lumen ().
Figure 1. Premature fusion of a phagophore with a lysosome (left) would not deliver the cargo into the lysosome lumen. STX17 (not depicted) only associates with the completed autophagosome. By preventing fusion until autophagosome completion (right), STX17 ensures that the cytoplasmic cargo is exposed to the degradative content of the lysosome.
Further analysis of this novel regulatory step disclosed the unique structure of STX17 that is necessary for its translocation to the autophagosome. Whereas the previously characterized mammalian Qa-SNARE proteins contain a single transmembrane domain (TMD) at their C terminus, STX17 has a unique hairpin-like structure formed by two TMDs, which exposes its C terminus to the cytosol. The TMDs contain the glycine zipper motifs that, when packed into the hairpin-like structure, expose hydrophobic amino acid residues to the hydrophobic interior of the membrane bilayer. The strict requirement of this structure for the localization of STX17 to autophagosomes, but not to the ER or the mitochondria, opens the question of the subcellular localization of this protein before its integration into the autophagy pathway; STX17 is less hydrophobic than typical TMD-containing SNARE proteins, and a large pool of STX17 is localized in the cytosol. In addition, it is not known how the unique STX17 structure participates in targeting this protein specifically to the autophagosome. Does another autophagosomal protein, or the curvature or lipid composition of the autophagosome play a role in this mechanism?
The manuscript by Itakura et al. provides an important clue for understanding the regulation of autophagosome-lysosome fusion, but some interesting questions remain to be addressed. For example, what controls the timing of STX17 incorporation into the autophagosome? Also, how is the fusion between the autophagosome and the vacuole regulated in yeast, which does not have a STX17 homolog? Answering these questions would further advance our understanding of the complex autophagy pathway.
Acknowledgements
This work was supported by NIH grant GM053396 to D.J.K.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012; 151:1256 - 69; http://dx.doi.org/10.1016/j.cell.2012.11.001; PMID: 23217709