Abstract
Phagophores engulf cytoplasmic material and give rise to autophagosomes, double-membrane vesicles mediating cargo transport to lysosomes for degradation. The regulation of autophagosome fusion with endosomes and lysosomes during autophagy has remained poorly characterized. Two recent papers conclude that STX17/syntaxin 17 (Syx17 in Drosophila) has an evolutionarily conserved role in autophagosome fusion with endosomes and lysosomes, acting in one SNARE complex with SNAP29 (ubisnap in Drosophila) and the endosomal/lysosomal VAMP8 (CG1599/Vamp7 in Drosophila). Surprisingly, a third report suggests that STX17 might also contribute to proper phagophore assembly. Although several experiments presented in the two human cell culture studies yielded controversial results, the essential role of STX17 in autophagic flux is now firmly established, both in cultured cells and in an animal model. Based on these data, we propose that genetic inhibition of STX17/Syx17 may be a more specific tool in autophagic flux experiments than currently used drug treatments, which impair all lysosomal degradation routes and also inactivate MTOR (mechanistic target of rapamycin), a major negative regulator of autophagy. Finally, the neuronal dysfunction and locomotion defects observed in Syx17 mutant animals point to the possible contribution of defective autophagosome clearance to various human diseases.
During autophagy, phagophores capture cytoplasmic cargo and form double-membrane autophagosomes, which are transport vesicles required for degradation of sequestered material in lysosomes; fusion with the degradative organelle is followed by recycling of the breakdown products. Autophagy is clearly becoming one of the hottest topics in biomedical research. Our understanding of the molecular machinery underlying autophagy made tremendous advances during the past 20 years, thanks to the discovery of autophagy-related (Atg) gene products in yeast. These evolutionarily conserved proteins are required for efficient autophagosome formation in all metazoan cells.Citation1,Citation2 Functional analyses of ATG genes revealed that autophagy has important roles in various developmental, physiological, and pathological processes.Citation1 Nevertheless, significant gaps have remained in our knowledge regarding autophagy. Among these are the process of autophagosome maturation, and the mechanisms that ensure timely and specific fusion with late endosomes and lysosomes.
SNAREs [soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor] are likely to be involved in autophagosome-lysosome fusion because the majority of vesicle fusion events are usually mediated by the action of these proteins. R SNAREs contribute an arginine (R) residue to the zero ionic layer of the assembled core complex, while Q SNAREs have a glutamine (Q) in the same position.Citation3 A trans-SNARE complex is formed by the binding of an R SNARE (associated with the first vesicle) to two or three proteins (located on the second compartment) that altogether supply three Q SNARE domains. After membrane fusion is accomplished, all four SNARE domains are bound to the same membrane and are referred to as a cis-SNARE complex. The approximately 30 SNARE proteins found in different organisms assemble in a combinatorial fashion, and the same protein is often incorporated in functionally distinct complexes, complicating mechanistic studies.Citation3 Clear loss-of-function phenotypes (ideally obtained with the use of null mutants and genetic rescue experiments), together with interaction and localization data for endogenous proteins, are key for proving the direct role of a given SNARE in autophagy.
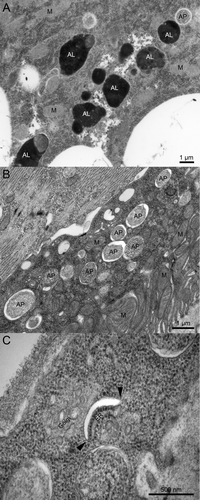
In yeast cells, autophagosomes can directly fuse with the vacuole, the functional counterpart of lysosomes. In contrast, delivery of autophagosomal contents to lysosomes in metazoan cells appears to depend on late endosomes.Citation4-Citation6 Furthermore, the SNAREs Vam3 and Vam7 that are required for autophagosome-vacuole fusion in yeast do not have clear homologs in animals, and no autophagosome-associated SNARE protein has been identified in metazoan cells until recently.Citation3,Citation7,Citation8 Two papers now reveal how the specificity of autophagosome fusion with endosomes and lysosomes is achieved.Citation9,Citation10 In one of these papers, we presented the results of a systematic screen for SNARE proteins involved in starvation-induced autophagy, using genetic mosaics of Drosophila larvae.Citation9 RNAi depletion of Syx17, ubisnap [the fly homolog of SNAP29 (synaptosomal-associated protein, 29 kDa)], and CG1599 [Vamp7 (vesicle-associated membrane protein 7)] leads to a similar, very characteristic phenotype: autophagosomes positive for Atg8a accumulate in the perinuclear region of fat body cells (the fly equivalent of hepatocytes and adipocytes). Autophagic degradation is completely blocked in these cells, as no LysoTracker-positive acidic autolysosomes form, no autophagy-dependent lysosomal quenching of GFP-Atg8a fluorescence is observed, and selective autophagic degradation of ref(2)P (mammalian SQSTM1/p62) aggregates is impaired. More rigorous genetic tests of Syx17 and CG1599/Vamp7 mutants show large-scale accumulation of autophagosomes in larval and adult tissues, based on commonly used transgenic reporters, immunofluorescent staining, western blot and ultrastructural analyses. . shows the morphology of typical double-membrane autophagosomes that abound in Syx17 mutant larval tissues, and also illustrates a phagophore, which is a rarely observed structure likely to be short-lived both in control and mutant cells. Importantly, these autophagy defects could be rescued by transgenic expression of the wild-type protein in Syx17 mutant backgrounds. In line with the results of our screen, Syx17 forms a complex with ubisnap and CG1599, the closest fly homolog of the mammalian VAMP7 and VAMP8 proteins. Finally, we showed that endogenous Syx17 is present in Atg8a-positive autophagosomes, based on immunofluorescence microscopy and immuno-electron microscopy (EM), but not in phagophores [marked by endogenous Atg5, or by Atg8a in Atg2 mutants that accumulate stalled phagophore assembly sites (PASs)].
Figure 1. Ultrastructural images of wild-type and Syx17 mutant Drosophila cells from early L3 stage larvae starved for 3 h. (A) Both autophagosomes (AP) and electron-dense autolysosomes (AL) can be observed in various tissues of starved control larvae, such as the fat body shown here. M, mitochondrion. (B) Typical double-membrane autophagosomes accumulate in large numbers in starved Syx17 mutant larvae, illustrated by a Malpighian tubule cell in this image. (C) This panel shows a rarely observed phagophore in a Syx17 mutant cell, which appears as a curved, nonbranching membrane cistern with a characteristic cleft between the two membranes after standard glutaraldehyde fixation. While the empty-looking space is an artifact, it greatly facilitates the identification of early autophagic structures. Arrowheads point to the edges of the phagophore, where the transitions of prospective outer and inner membrane layers are clearly visible.
In the other study, siRNA silencing of STX17 in cultured human cells was also found to result in the accumulation of autophagosomes positive for LC3 (a human homolog of Atg8), and this phenotype could be rescued by co-transfection with an RNAi-resistant transgene.Citation10 This paper also identified a SNARE complex of STX17 (Qa), SNAP29 that contains two SNARE domains (Qbc), but no membrane anchor, and lysosomal/endosomal VAMP8 (R). As expected from the interaction data, silencing of either SNAP29 or VAMP8 phenocopied the loss of STX17. Moreover, GFP-Syntaxin 17 could be detected in LC3-positive autophagosomes, but not in phagophores, and immuno-electron microscopy suggested that it is located in the outer membrane of double-membrane autophagosomes. These data support the following model for fusion: STX17 is first loaded onto the outer bordering membrane of autophagosomes and recruits SNAP29, which is followed by binding to VAMP8 (CG1599/Vamp7 in flies) located in endosomes and lysosomes. After fusion is completed, the cis-SNARE complex is presumably disassembled and recycled from the lysosomal membrane through the actions of NSF (N-ethylmaleimide-sensitive factor) and NAPA (NSF attachment protein, α, also known as α-SNAP), as usual.Citation3
These two recent papers shed some light onto how autophagosomes gain competence for fusion with endosomes and lysosomes, as phagophores never participate in such events. A new question immediately arises: how is STX17/Syx17 transported to the outer membrane of autophagosomes? Itakura et al. found that a free cytoplasmic pool of STX17 exists, which is likely to be the result of its atypical structure.Citation10 Unique among SNARE proteins, it contains two transmembrane domains. These glycine-rich motifs were suggested to form a glycine zipper, mediating close packing of the two domains. Interestingly, replacement of selected glycines with leucines greatly reduces the autophagosomal localization of this protein without affecting its presence in the ER and mitochondria. The specific mechanism responsible for loading STX17 into the outer autophagosomal membrane only after sealing of the phagophore is not yet known. Future studies should provide further insights into this process, such as by proteomic identification of STX17 binding partners, or through the analysis of new hits from genetic screens that result in autophagosome accumulation by preventing STX17 recruitment to autophagosomes.
The number and size of autophagic structures at a given time point depends on two processes: the rate of autophagosome formation (incoming material), and degradation of sequestered cargo in lysosomes (outgoing material). Thus, experiments aimed at measuring autophagic flux have become an essential part of autophagy analyses. The most common test for this purpose is the application of bafilomycin, chloroquine, or similar drugs that block lysosomal breakdown.Citation11 Unfortunately, these treatments also impair degradation of endosomal cargo and interfere with lysosome-dependent activation of MTOR, a major regulator of cell growth and autophagy.Citation12,Citation13 In addition, the use of bafilomycin is limited to cultured cells due to toxicity in complex multicellular organisms. Although a role of STX17 in endocytosis has not been excluded yet, we propose that mutation or RNAi knockdown of this gene may eventually turn out to be more specific than bafilomycin treatment in autophagic flux experiments. Importantly, it can also be used to determine autophagic flux in whole animals in addition to cultured cells.
A third recent study suggested that autophagosomes may often form in the vicinity of both the ER and mitochondria, and implicated a role for STX17 in phagophore closure based on limited analysis.Citation14 Many aspects of the two human cell culture reports match: STX17 was found on both mitochondria and the ER, LC3-positive dots accumulate in microscopy images of STX17-depleted cells, and the levels of lipidated LC3 and the selective autophagy cargo SQSTM1 increased in western blots of siRNA cell lysates.Citation10,Citation14 It is important to highlight that these tests do not distinguish between accumulation of phagophores or autophagosomes. Unfortunately, multiple experiments performed in these different labs produced contradicting results.Citation10,Citation14 First, Itakura et al. found no colocalization of STX17 with numerous markers of the PAS including ATG14, ATG16L1, ZFYVE1/DFCP1, ULK1 or WIPI1, whereas Hamasaki et al. reported its partial colocalization with ATG14, but they did not test other PAS markers. Second, Itakura et al. detected about half of the endogenous (or GFP-tagged) STX17 pool in the cytosolic fraction after ultracentrifugation, whereas Hamasaki et al. showed that endogenous STX17 is almost entirely membrane-associated, and it is hardly detectable in the cytosol in fractionation experiments. Transmission EM is still one of the most informative autophagy tests, and helps to interpret results obtained by other methods. The prerequisite of reliable support by transmission EM is the exact and rigorous identification of the various autophagic structures. Itakura et al. detected and quantified the accumulation of typical double-membrane autophagosomes in STX17 siRNA cells. In the ultrastructural images selected by Hamasaki et al., the identity of phagophores and autophagosomes is uncertain in many cases even in enlarged images due to low resolution, tangentional sectioning and discontinuity of the membranes at certain places.
Nevertheless, these studies are not entirely incompatible with each other. It could be possible that STX17 is also involved in autophagosome formation, although it is not clear why unsealed phagophores with a size of regular autophagosomes would accumulate if the allegedly critical membrane trafficking from ER and mitochondria was blocked with STX17 siRNA. One may argue that the siRNA treatments by Hamasaki et al. were more potent than those of Itakura et al., and perhaps revealed an earlier function. Careful biochemical and cell biology analysis of chromosomally deleted alleles (knockouts) of STX17 in mouse embryonic fibroblasts and in mice will be very important for resolving these discrepancies. Our Drosophila studies could also be complicated by the presence of maternally provided Syx17 gene products: a 40-kDa isoform of unknown identity is still visible and only the main, 34-kDa isoform is missing in mutant larvae, although neither isoform can be detected in western blots of adult lysates. The large-scale accumulation of autophagosomes in neurons of Syx17 mutant adults, occupying 20% of the cytoplasm in the perikaryon on average vs. 0% in control or genetically rescued animals, strongly suggests that Syx17 is dispensable for autophagosome formation in Drosophila. Still, mutants lacking both maternal and zygotic gene products can be analyzed to exclude maternal contribution in all developmental stages in flies.
In addition to serving cytoprotective roles during stress and starvation, autophagy is thought to be required for proper homeostasis under normal conditions.Citation1 This function seems to be especially critical in long-lived cells such as neurons. Supporting this view, we and others have reported that loss of basal autophagy leads to progressive accumulation of ubiqutinated protein aggregates and neurodegeneration, both in fly mutants of Atg7 and in mice with neuron-specific deletion of Atg5 or Atg7.Citation15-Citation17 Different from these previous studies of Atg mutations that prevent autophagosome formation, Syx17 mutation leads to large-scale accumulation of autophagosomes in neurons of adult flies.Citation9 These mutants perform very poorly in a climbing assay, a standard test for neuronal dysfunction, and all die within 4 d of eclosing from the pupal case, in contrast with normal flies that live for months. Since genetic suppression of cell death in neurons does not improve these mutant phenotypes, unlike transgenic expression of wild-type Syx17, it is likely the accumulation of autophagosomes that renders these neurons unable to perform their normal tasks.Citation9 The first animal model of genetically impaired autophagosome fusion clearly shows that in addition to the initial sequestration of cytoplasmic cargo, the final lysosomal breakdown is also essential. Numerous diseases are characterized by the accumulation of autophagosomes and autophagic vesicles, such as neurons in Alzheimer disease and muscles in vacuolar myopathy patients, respectively.Citation1 Therefore, it will be exciting to see whether future studies can link genetic defects of the STX17-dependent fusion machinery to certain human diseases.
Acknowledgments
Work in the Juhasz lab is funded by the Wellcome Trust (087518/Z/08/Z) and the Hungarian Scientific Research Fund (OTKA K83509).
Disclosure of Potential Conflicts of Interest
The authors declare that no financial or other conflicts exist.
References
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069 - 75; http://dx.doi.org/10.1038/nature06639; PMID: 18305538
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27:107 - 32; http://dx.doi.org/10.1146/annurev-cellbio-092910-154005; PMID: 21801009
- Hong W. SNAREs and traffic. Biochim Biophys Acta 2005; 1744:493 - 517; http://dx.doi.org/10.1016/j.bbamcr.2005.03.014; PMID: 16038056
- Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM, et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol 2007; 179:485 - 500; http://dx.doi.org/10.1083/jcb.200702115; PMID: 17984323
- Rusten TE, Vaccari T, Lindmo K, Rodahl LM, Nezis IP, Sem-Jacobsen C, et al. ESCRTs and Fab1 regulate distinct steps of autophagy. Curr Biol 2007; 17:1817 - 25; http://dx.doi.org/10.1016/j.cub.2007.09.032; PMID: 17935992
- Juhász G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 2008; 181:655 - 66; http://dx.doi.org/10.1083/jcb.200712051; PMID: 18474623
- Moreau K, Renna M, Rubinsztein DC. Connections between SNAREs and autophagy. Trends Biochem Sci 2013; 38:57 - 63; http://dx.doi.org/10.1016/j.tibs.2012.11.004; PMID: 23306003
- Wang CW, Stromhaug PE, Shima J, Klionsky DJ. The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways. J Biol Chem 2002; 277:47917 - 27; http://dx.doi.org/10.1074/jbc.M208191200; PMID: 12364329
- Takáts S, Nagy P, Varga A, Pircs K, Kárpáti M, Varga K, et al. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J Cell Biol 2013; 201:531 - 9; http://dx.doi.org/10.1083/jcb.201211160; PMID: 23671310
- Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012; 151:1256 - 69; http://dx.doi.org/10.1016/j.cell.2012.11.001; PMID: 23217709
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445 - 544; http://dx.doi.org/10.4161/auto.19496; PMID: 22966490
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011; 334:678 - 83; http://dx.doi.org/10.1126/science.1207056; PMID: 22053050
- Juhász G. Interpretation of bafilomycin, pH neutralizing or protease inhibitor treatments in autophagic flux experiments: novel considerations. Autophagy 2012; 8:1875 - 6; http://dx.doi.org/10.4161/auto.21544; PMID: 22874642
- Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013; 495:389 - 93; http://dx.doi.org/10.1038/nature11910; PMID: 23455425
- Juhász G, Erdi B, Sass M, Neufeld TP. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev 2007; 21:3061 - 6; http://dx.doi.org/10.1101/gad.1600707; PMID: 18056421
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885 - 9; http://dx.doi.org/10.1038/nature04724; PMID: 16625204
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441:880 - 4; http://dx.doi.org/10.1038/nature04723; PMID: 16625205