Abstract
A high MAPK1/3 (also known as ERK2/1, respectively) activity, preventing spontaneous activation, is essential to maintain cell cycle arrest of mature oocytes of mammals, frogs or invertebrates such as starfish. Mature oocytes would undergo a “suicide”-like cell death if not fertilized. We previously have reported that downregulation of MAPK1/3 in unfertilized sea urchin eggs induces a calcium-dependent entry into mitosis. We show here that this event is followed by a series of pseudo-mitotic cell cycles associated with transient Cai increases, preceding CASP3/caspase-3 activation and apoptosis. However, cell death was delayed after inhibition of the Cai transients or of cyclin-dependent kinases (CDK), with roscovitine. In these conditions, eggs enter an autophagy program as suggested by detection of processed LC3B by western blot, immunofluorescence and immunogold staining, visualization of autophagy vesicles by electron microscopy, and an increase in acidic vesicular organelles (AVOs). We found that bafilomycin A1 or an association of leupeptin and pepstatin, which are widely used to study autophagy, may act upon calcium signaling or cell cycle events, respectively, and not only on autophagy events. Finally, inhibition of PtdIns 3-kinase with wortmannin or LY294002 powerfully stimulated cell death of unfertilized eggs, which suggests that this activity does not negatively regulate autophagy as is often reported, but rather stimulates survival in unfertilized eggs. We suggest that apoptosis of unfertilized eggs is the consequence of an aberrant short attempt of development that occurs if MAPK1/3 is inactivated, but these eggs can use autophagy as a survival program when the cell cycle is blocked.
Introduction
After completion of meiosis, oocytes are arrested at a stage that differs with the species and during which fertilization can occur. In mammalian eggs, fertilization must occur during an optimal period after which fertilization of the egg triggers apoptosis rather than development.Citation1,Citation2 Furthermore, mature eggs from mammals, frogs or invertebrates such as Drosophila or starfish, ultimately undergo suicide-like cell death if not fertilized after a period of time that differs with the species.Citation2,Citation3 This period, during which fertilization triggers proper development, is less than 10 h in mammalian oocytes (around 14 h in starfish eggs, whereas sea urchin eggs can be kept for days before being fertilized).Citation2,Citation4 One question that arises concerns the type of cell death that is adopted by the egg. A Nomenclature Committee on Cell Death has very recently proposed a guideline that lists the different types of cell deaths, including apoptosis, necrosis, autophagy or mitotic catastrophe.Citation5,Citation6 Death in unfertilized mammalian oocytes,Citation7 starfish eggsCitation8,Citation9 and sea urchin eggsCitation10 is generally considered as apoptosis due to the presence of signaling pathways characteristic of this cell death program. Signs of autophagy have also been observed in mammalian oocytesCitation7 and insect eggs.Citation11 However, causal correlation among different cell death pathways that may take place in unfertilized eggs remains to be determined and stimuli causing the activation of these pathways in vitro and in vivo also need to be clarified. Moreover, although autophagy has been recently detected in sea urchin embryos as a survival process against cadmium stress,Citation12 it is not known whether unfertilized eggs of this species can also use autophagy for survival.
The MAP2K-MAPK1/3 (MEK-ERK) signaling pathway has been widely described to play major roles in the regulation of cellular processes as diverse as proliferation, differentiation, development, learning, survival or cell death. How this cascade can be involved in so many distinct and even opposing processes is far from being fully understood. Duration and strength of MAPK1/3 activation, changes in subcellular localization or interactions with other signaling pathways determine cell fate.Citation13 The MOS-MAP2K-MAPK1/3 cascade is also well known to regulate meiosis of oocytes.Citation14 After completion of meiosis, a high MAPK1/3 activity seems to be essential to maintain cell cycle arrest in G1 arrested unfertilized starfishCitation15 or jellyfish eggs,Citation16 as well as metaphase I arrest in cnidarianCitation17 or ascidianCitation18 eggs, and metaphase II arrest in vertebrate oocytes.Citation19,Citation20 In all these species, inactivation of MAPK1/3 induced by fertilization or after artificial inhibition of MOS or MAPK1/3 triggers entry into mitotic M phase. An active MOS-MAPK1/3 pathway after meiosis I would then prevent these oocytes to progress through embryonic mitotic cycles and would be required for fertilization to occur. It is interesting to note that a steady decline of maturation-promoting factor (MPF) and MAPK1/3 has been associated with post-ovulatory aging of mammalian oocytes.Citation2 In G1 arrested unfertilized starfish eggs, two successive phases would lead to cell death, the first phase requiring an active MAP2K-MAPK1/3 cascade followed by a second one requiring MAPK1/3 inactivation for apoptosis to occur.Citation9,Citation21 The situation in sea urchin appears more conflicting. According to all of these results, our previous work showed that unfertilized sea urchin eggs also contain a high MAPK1/3 activity that is inactivated after fertilization,Citation22,Citation23 but other authors detected in these eggs an MAPK1/3 protein that is not active.Citation24 We also previously reported that artificial inactivation of MAPK1/3 triggers entry into M phase as in other species,Citation25 but it was not known whether this condition also induced progress into embryonic development as described above for starfish or ascidian. All these data prompted us to investigate in the sea urchin model how the MAPK1/3 signaling cascade may link cell cycle progression and the triggering of cell death in unfertilized eggs.
Finally, variations in free cytosolic calcium concentration (Cai) are well known to trigger not only key cellular functions but also to regulate mitotic divisions in numerous cell types including eggs.Citation26 Our previous data showed that artificial inactivation of MAPK1/3 in unfertilized sea urchin eggs triggers a decrease in the inositol 1,4,5-trisphosphate (InsP3) sensitivity but an increase in intracellular calcium (Cai).Citation25 Fine tuning of Cai by well-regulated endoplasmic reticulum-mitochondria cross-talk is a major control mechanism of life and death that has been described in many cell types.Citation27 Here we have investigated the implication of Cai homeostasis during cell death of the unfertilized oocyte. Altogether, this study deciphers how the MAP2K-MAPK1/3 pathway and mitotic progression by cyclins and CDKs and Cai control the fate of unfertilized eggs: death by apoptosis vs. survival by autophagy.
Results
Inactivation of the MAP2K-MAPK1/3 cascade in unfertilized eggs leads to mitotic cycles correlated with Cai peaks preceding egg fragmentation
We previously reported that treatment during 3 to 4 h of unfertilized eggs with 1 to 5 µM U0126, a widely used MAP2K inhibitor, induced a rapid decrease in P-MAPK1/3 followed by MPF activation and nuclear envelope breakdown (NEB), indicating entry into mitosis.Citation25 We investigated whether these eggs would be capable of entering embryonic development. Five eggs were recorded for longer durations under a video-microscope; one is shown (). , i indicates that the nucleus of this egg (, i, part a) disappeared (, i, parts b, d, f, h, i, k) and then reformed (, i, parts c, e, g, j, i). In addition, a series of cytoplasmic contractions followed by recovery of a round shape (relaxation) started after one or several rounds of NEB had occurred (, i, parts i, j) and ended in egg fragmentation (, i, part l). These constrictions always occurred at times of NEB or during reformation of the nucleus (, ii). Four eggs showed 6 cycles of NEB as shown in , ii whereas one egg showed 3 successive rounds of NEB (not shown). Analysis of the time between successive sequences of NEB () and of constrictions () shows a period that was constant for a given egg population, strongly suggesting that these eggs entered a program of mitotic cycles. These U0126-treated eggs failed to complete either karyokinesis or cytokinesis but oscillated fairly regularly between a mitotic-like state and an interphase-like state. In eight similar experiments, all eggs were fragmented 7 to 18 h after addition of 5 µM U0126, depending on the batch of eggs. None of these events was seen after treatment of the eggs with the inactive compound U0124, and as already observed.Citation22,Citation23,Citation25
Figure 1. MAPK1/3 inactivation in unfertilized eggs triggers cycles of NEB and egg constrictions linked to Cai variations that precede egg fragmentation. (A) Morphology of one egg treated with 5 µM U0126. (i) DIC images. Time (bottom left) is given in min after U0126 addition (t = 0). (ii) Time course of nuclear appearance (dark gray) and contractions occurrence (light gray). 0 and 1 are arbitrary values indicating absence of both nucleus and constriction (round cell, given value = 0) and clear nucleus or deep constriction (given value = 1). (B) Correlation between constrictions and Cai changes. (i) Image sequence (see Vid. S1) starting at time 320 min after U0126 addition. Time between 2 images shown is 4 min. (ii) Variations of Cai levels in the egg shown in (Bi). Sequences of contraction (indicated by arrows)/relaxation were determined as in (A). (iii) Comparison between a common fertilization Cai signal recorded after sperm addition (Fert) and an example of Cai bursts occurring between 340 and 550 min after addition of 5 µM U0126.
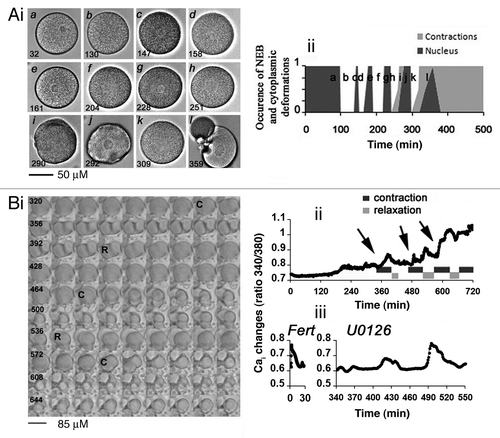
Table 1. Analysis of NEB induced by MAP2K/MEK inhibition
Table 2. Analysis of contraction cycles induced by MAP2K/MEK inhibition
We previously showed that inactivation of MAPK1/3 induces an increase in Cai in the 3 h following U0126 treatment.Citation25 The morphology and the Cai changes recorded during a long time in one egg representative of 6 eggs (see Vid. S1) injected with fura-2 and treated with U0126 are shown in , i and ii, respectively. A series of 4 sequences of contraction (C)/relaxation (R) was clearly visualized in this egg (, i), with constrictions occurring at times of Cai increases (, ii). In addition, a long-lasting steady increase in Cai level superimposed onto the Cai transients was measured in all eggs (, ii). These changes in Cai were reflected in a more or less elevated fertilization membrane as previously observedCitation25 and could reach an amplitude similar to that measured during the fertilization Cai signal (, iii). No clear Cai variations occurred in untreated eggs during the same time of recording (not shown). In this experiment, 5 eggs were fragmented 12 h after U0126 treatment while one egg remained not fragmented at that time (not shown). In conclusion, the cell death pattern observed in unfertilized eggs deprived of MAPK1/3 activity may be the consequence of the lack of parthenogenesis as proper cell division was never observed.
MAPK1/3 inactivation induces a calcium-dependent apoptotic pathway involving CASP3 activation
We questioned whether the Cai changes occurring after MAPK1/3 inactivation were necessary to trigger cell death. Ca/EGTA buffer was injected at a concentration capable of inhibiting the fertilization Cai waveCitation25 and the Cai changes normally recorded in eggs treated with U0126 (not shown). , i shows that one egg injected with EGTA and treated with U0126 kept a round shape for at least 500 min and then fragmented at 607 min, while the noninjected egg showed constrictions followed by fragmentation occurring 390 min after U0126 addition. Injection of EGTA buffer prior to U0126 treatment induced either inhibition or delay of NEB (not shown) and delayed but did not inhibit fragmentation of U0126-treated eggs (, ii).
Figure 2. Inactivation of MAPK1/3 triggers a Ca-dependent apoptotic pathway with stimulation of CASP3. (A) EGTA buffer injection delays egg fragmentation induced by 5 µM U0126. (i) Images chosen at representative times (min noted in white) taken from a movie of 2 eggs treated with U0126 and injected with or without EGTA buffer (white asterisk). (ii) Time course of fragmentation after injection (7 eggs) or no injection (10 eggs) of EGTA in eggs from the same population treated with 5 µM U0126. Two experiments gave similar results, one is shown. (B) Time course of CASP3 activity after treatment of unfertilized eggs with 5 µM U0126. Representative images of eggs at the indicated time points are displayed around the graph.
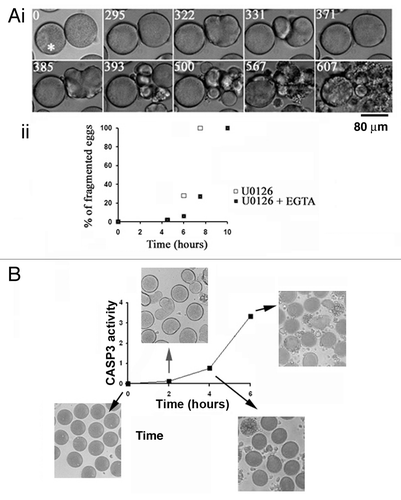
We found that CASP3 was stimulated before complete egg fragmentation but well after the beginning of the constriction/relaxation series that began around 3 h after U0126 addition in the experiment shown in .
These results suggest that an apoptotic cell death program occurring after MAP2K-MAPK1/3 inactivation is promoted by a Cai dependent entry into mitosis.Citation25
Preventing entry into mitosis delays cell death in eggs deprived of MAPK1/3 activity
We next tested whether inhibiting MPF and therefore mitosis entryCitation28 could alter egg fragmentation in these conditions. We used roscovitine, an inhibitor of CDKs (and hence of MPF) that binds the ATP binding pocket of CDK1/CDC2 and therefore inhibits activity but not activation of CDK1.Citation29 This inhibitor was used at the concentration of 20 µM, which has been widely used in the sea urchin model to cancel MPF activity.Citation24,Citation30,Citation31
As shown for two different batches of eggs (, i), unfertilized eggs could be kept and remained fertilizable for 9 to 30 h at room temperature (18 to 21°C), depending on the egg population (n = 8). In 5 egg populations, roscovitine delayed fragmentation that normally occurs in aging eggs (, i and ii). It also prevented NEB as expected and delayed fragmentation that occurs in eggs treated with U0126 (, i and ii).
Figure 3. Roscovitine delays fragmentation of unfertilized eggs whether or not they are deprived of MAPK1/3 activity. Unfertilized eggs were not treated (Control, C) or treated with 2 µM U0126 (U), 20 µM roscovitine (R), or with both inhibitors (UR). (A) Fragmentation. (i) time course in two different batches of eggs (1 and 2); (ii) images from experiment 1 of unfertilized eggs taken at time 0, 10 or 14 h after treatment. Only eggs showing clear huge blebs and extrusions of cytoplasm were counted as fragmented (indicated with a star in ii). (Bi) P-MAPK1/3 levels; (ii) P-CDK1(Tyr15) levels. Western blot (left panels) using anti-P-MAPK1/3 antibody or anti-P-CDK1(Tyr15) antibody and quantification of signals (right panels).
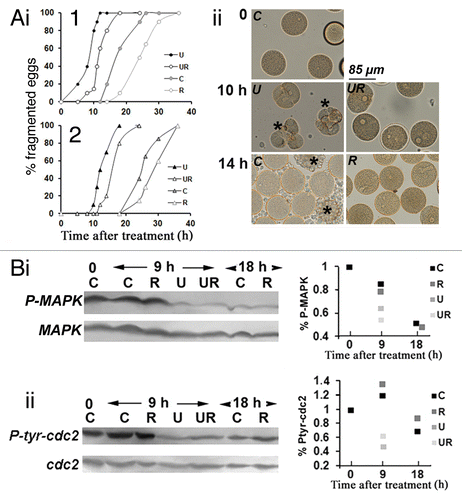
It has been suggested that aged unfertilized mammalian eggs die because of a decline in P-MAPK1/3 occurring over time.Citation2 Unfertilized spawned sea urchin eggs contain a high level of P-MAPK1/3 (, i) and of P-CDK1(Tyr15) (, ii). Eggs that could still be fertilized 9 h after spawning contained a high level of P-MAPK1/3 (, i). Beyond that time, these eggs began to fragment (, i) while the level in P-MAPK1/3 decreased slowly to reach, 18 h after spawning, a low level similar to that seen in U0126-treated eggs (, i). Roscovitine neither affected the inactivation of P-MAPK1/3 that occurred in aging eggs nor that measured in U0126-treated eggs (, i). Similarly, P-CDK1(Tyr15) did not change in untreated eggs kept for 9 h but decreased after 18 h in the presence or absence of roscovitine (, ii). As already reported,Citation25 P-CDK1(Tyr15) was reduced after U0126 treatment, which was still observed in the presence of roscovitine (, ii).
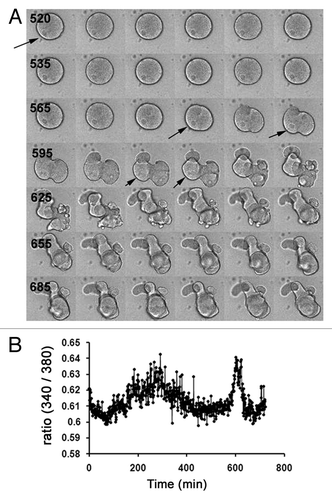
Finally, Cai fluctuations still occurred in eggs treated with both 20 µM roscovitine and 5 µM U0126. One egg is shown as an example in . This egg kept its round shape until 575 min and showed movements of the nucleus, which remained intact until at least 610 min, after which fragmentation occurred (). Therefore, this egg did not undergo NEB/NE reformation cycles and no sequences of mitotic events were observed (). A large Cai peak lasting 400 min could be observed, followed at 565 min by a second sharper Cai increase associated with strong constrictions ().
Figure 4. Roscovitine does not inhibit Cai changes induced by MAPK1/3 inactivation in unfertilized eggs. (A) Image sequence from 505 to 710 min showing changes in the morphology of a representative egg injected with fura-2 and treated with 5 µM U0126 and 20 µM roscovitine. Time between 2 images is 5 min (time displayed in black). This egg showed movements of the nucleus which remained until at least 610 min (arrows) and kept its round shape until 575 min. (B) Changes in Cai in the egg shown in (A).
Altogether, this first set of results suggests that cycling in and out of M phase facilitates fragmentation of unfertilized sea urchin eggs after inactivation of MAP2K-MAPK1/3 pathway.
Unfertilized eggs show signs of autophagy after MAPK1/3 inactivation when CDKs activities are also suppressed
The fact that eggs deprived of MAPK1/3 and CDK activities did not enter mitosis and fragmented with a delay led us to investigate whether autophagy, which is both a survival and a cell death program, could be triggered in these eggs. We followed a standard set of criteria to monitor this process.Citation32 We first checked whether cleavage and lipidation of LC3B, which indicates formation of autophagosomes, could be detected.Citation33 A western blot analysis is shown in , i. In this experiment, aging eggs died 28 h after spawning while 50% of eggs fragmented 14 and 22 h after U0126 or U0126 + roscovitine treatment, respectively. Eggs were also treated with 2 µM staurosporine which has been described to induce apoptosis in sea urchin eggsCitation10 and induced fragmentation 9 h after treatment in this population of eggs. Both LC3B-I and the cleaved and lipidated lower MW form of LC3B (LC3B-II) could be detected in unfertilized eggs in similar proportions in control eggs, aging control eggs, eggs treated with roscovitine or with staurosporine. Although a slight increase in LC3B-II was detected in eggs treated during 10 to 15 h with U0126 only, a large increase in the proportion of this band appeared in eggs treated for 20 h with U0126 and roscovitine. Three experiments were performed that gave similar results (, ii).
Figure 5. Survival of eggs deprived of MAPK1/3 and CDK activities involves LC3B. (A) LC3B cleavage. (i) Western blot analysis with anti-LC3B Ab (upper panel) and internal control with anti-TUBA (lower panel) with quantification of bands (histograms). Eggs were not treated (control eggs, C) or treated with staurosporine (St), U0126 (U) or roscovitine (R) only or with both U0126 and roscovitine (UR) for 7, 10, 12 or 20 h. The lower bands of the gel (LC3B-II) and both bands (total LC3B) have been quantified as explained in Materials and Methods. (ii) Changes in the proportion of LC3B-II. Three experiments that gave similar results were pooled. Percentage of LC3B-II (lower band) as visualized in (i) is here expressed relative to the total amount of LC3B arbitrarily taken as 100. Signals are obtained in control eggs (C, time zero), eggs treated during 10 to 13 h with U0126 only (U), or eggs treated during 17 to 20 h with roscovitine only (R) or with U0126 and roscovitine (UR). In the three experiments, eggs treated with U0126 only died within 16 h treatment. Only values (means ± sem) obtained in UR eggs are significantly different from those of C eggs (Student test, P < 0.01). (B) LC3B cytoplasmic distribution. (i) Observation of eggs treated for 10 h with U0126 only (U) or with U0126 and roscovitine for 18 h (UR) after fixation and immunolabeling with the anti-LC3B Ab. An image by transmitted light microscopy (left panels) and a z projection of five images taken from a stack of images obtained by confocal microscopy and localized in the center of the egg (U) or around the nucleus (UR) (right panels) are shown. An enriched particulate distribution of LC3B (arrow) around the nucleus (star) was only seen in UR eggs. (ii) Variation in the amount of large LC3B structures. Particles larger than 2.95 µm2 were quantified as explained in Materials and Methods. Values (means ± sem, 10 eggs/condition) obtained UR eggs are significantly different from those of C eggs (Student test, P < 0.01). (C) Effect of injection (arrow) of the anti-LC3B antibody before treatment of unfertilized eggs with U0126 only (i) or with both U0126 and roscovitine (ii).
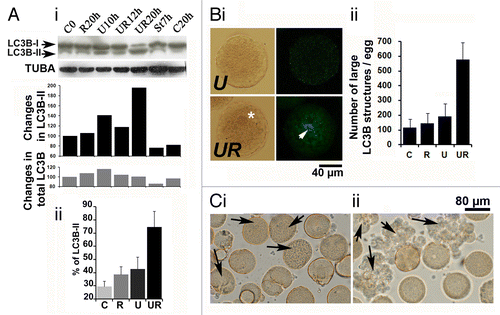
Observation by confocal microscopy after immunohistochemistry showed that cytoplasmic distribution of LC3B was diffuse and hardly detectable in control eggs and in eggs treated with roscovitine only (not shown). A few LC3B patches were detected after U0126 treatment (, i), and a more heavily fluorescent particulate distribution of LC3B was seen in eggs treated with U0126 and roscovitine (, i). Quantification of images indicate that the amount of large LC3B structures was slightly increased in eggs treated by U0126 only and was largely enhanced in eggs treated with the two inhibitors (, ii).
In order to investigate the role of LC3B, we injected the anti-LC3B antibody in eggs before treatment. We found that entry into mitosis, the appearance of contractions and the fragmentation of 27 eggs treated with U0126 occurred with the same frequency with or without injection of the antibody (, i). On the contrary, 32 eggs treated with both U0126 and roscovitine died very quickly after injection of the antibody (, ii), which suggests that LC3B and formation of autophagosomes were crucial steps in the survival of eggs.
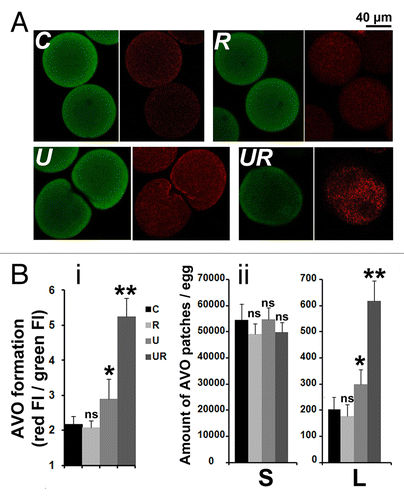
To identify the development of acidic vesicular organelles (AVOs), which is characteristic of autophagy, we used the lysosomo-tropic agent acridine orange (AO) that diffuses across biological membranes when uncharged.Citation34,Citation35 Its protonated form accumulates in acidic compartments where it forms aggregates that fluoresce red. indicates that bright red fluorescent puncta accumulated around the nucleus of eggs treated with U0126 and roscovitine, while the fluorescence remained diffused in the cytoplasm in control eggs or eggs treated with U0126 only. The global red fluorescence (, i) and the amount of AVO patches of a size larger than 2.95 µm2 (, ii) were significantly increased in eggs treated with U0126 only and largely enhanced in eggs treated with U0126 and roscovitine, while the small size AVO patches were similarly distributed in all conditions (, ii).
Figure 6. Roscovitine increases the amount of AVOs in U0126-treated eggs. (A) Visualization of AVOs by confocal microscopy. C, R, U, and UR are as described in . A z projection of five images taken from a stack of images and localized in the center of the egg (U) or around the nucleus (C, R, and UR) are shown. AO accumulates in eggs as seen by the green color detected in the three conditions (left panels), and fluoresces in red (right panels) as an indication of acidity. Note that AO enters the large nucleus of UR eggs but without showing high intensity of red fluorescence. (B) Quantification of AVOs. (i) Global fluorescence; (ii) amount of AVO patches of size smaller (S) or larger (L) than 2.95 µm2. Values (means ± sem, 12 eggs/condition) are significantly different (Student t-test, *P < 0.05 or **P < 0.01) or not significantly different (ns) from those of control eggs.
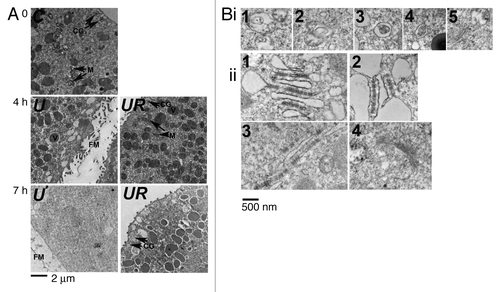
We then looked for the presence of autophagic vesicles by EM observation.Citation36 Pellets of resin-embedded eggs were cut into ultra thin slices. Each slice contained several sections of different sizes from eggs that have been cut at different levels of depth (an entire medium section for a 90 µm diameter egg being around 6000 µm2). Typical examples of eggs are shown in . Eggs treated with U0126 only (U) showed, after 4 h treatment, a cytoplasm that appeared less dense than that of control eggs (C) but that still contained various intracellular compartments including mitochondria and yolk platelets. The absence of cortical granules in these eggs corroborates the presence of a fertilization envelope. No clear intracellular structure could be observed after 7 h U0126 treatment. On the contrary, eggs treated with U0126 and roscovitine (UR) resembled control eggs after 4 h treatment and still contained various intracellular organelles after 7 h treatment. We screened 2 to 3 areas/egg (1800 to 3200 µm2) of sections from 7 different eggs treated for 7 h with U0126 and roscovitine and found a total of 21 zones (12 µm2 images) containing membrane-enclosed structures that looked like autophagosomes as those shown in , i. Two to 3 areas/egg from sections of 5 control eggs, or 4 eggs treated for 7 h with U0126 or 5 eggs treated for 7 h with roscovitine showed only rare autophagic structures, since a total of 3, 0 and 2 zones containing these structures were found, respectively (not shown). We also observed that eggs treated with U0126 and roscovitine contained endoplasmic reticulum (ER) that appeared much dilated (, ii, and ), indicating aberrant rearrangement of the ER in these conditions. These figures of dilated ER could not be due to fixation artifacts since other areas of the same eggs contained ER (, ii, ) or Golgi (, ii, ) that did not appear dilated. Furthermore, they were not seen in control eggs and in eggs treated with U0126 alone (not shown).
Figure 7. Roscovitine leads to the formation of autophagic vacuoles in U0126-treated eggs. (A) EM observation. C, R, U and UR are as described in . Eggs were either untreated (0) or treated for 4 h or 7 h. Cytoplasm of eggs treated for 7 h with U0126 and roscovitine (UR) retained the general aspect seen in control eggs with visible intracellular compartments including mitochondria (M), vitellin platelets (V) or cortical granules (CG). On the contrary, U0126 treated eggs showed elevation of a fertilization membrane (FM) after 4 h and no clear intracellular structure was visible 7 h after treatment. Eggs treated with roscovitine only appeared as per the control eggs (not shown). (B) Observation at higher magnification of eggs treated for 7 h with U0126 and roscovitine. (i) Five examples (1 to 5) of membrane-enclosed structures that resemble autophagosomes. (ii) Various areas containing ER that appeared very dilated (1 to 2) or containing undilated ER (3) or Golgi (4).
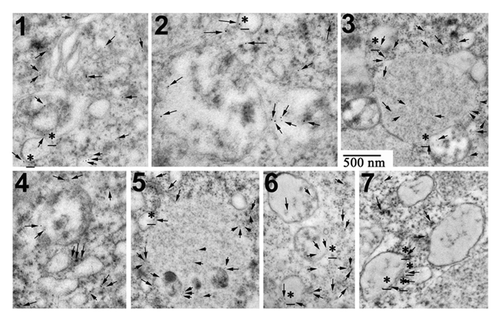
Finally, we performed immunogold labeling on similar EM ultra thin sections. We screened 2 to 3 areas/egg (2100 to 3200 µm2) from sections of 5 different eggs treated for 7 h with U0126 and roscovitine. 43 zones (12-µm2 images) were found containing 1 to 32 immunogold particles (11 ± 7, men ± sem, n = 43). Examples of labeled areas are shown in . Immunogold particles were consistently found in areas containing several adjacent vesicular structures, and were either at the closed periphery or associated with the membranes of these compartments. On the contrary, only scarce labeling was detected in control eggs or roscovitine-treated eggs (5 screened areas in each condition, 2400 to 2900 µm2, 10 to 15 immunogold particles total/area), and no labeling was detected in U0126-treated eggs.
Figure 8. LC3B is detected by immunogold labeling in eggs treated with U0126 and roscovitine for 7 h. (Examples 1 to 7) of images obtained after observation under EM are shown. Immunogold particles (arrow) were always detected close to vesicular structures that were associated together (3, 5, 7) and often localized in the vicinity of ER (1). Some particles were clearly associated with membranes of these structures (stars).
All these results suggest that an autophagy program is stimulated in unfertilized eggs where MAPK1/3 and CDKs are inactivated.
Leupeptin increases survival whereas Baf and PtdIns 3-kinase inhibitors stimulate cell death in unfertilized eggs
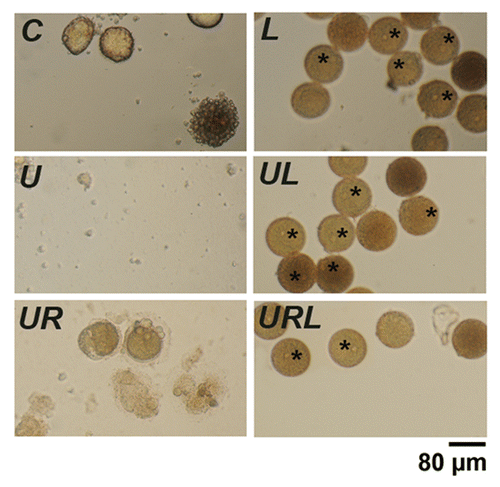
We tested the effect of a combination of leupeptin, a cathepsin B inhibitor, and pepstatin, a potent inhibitor of aspartyl proteases, that both impair amphisome-lysosome fusion and are commonly used to assess autophagic flux.Citation37 shows that naturally aging eggs (C) and eggs treated with U0126 alone (U) or with U0126 and roscovitine (UR) died after 7, 11, 21 h respectively. Addition of 20 µM leupeptin and 7 µM pepstatin to these eggs (L, UL and URL) very potently delayed fragmentation in the three conditions, since all eggs of this population remained intact after 26 h, showing an intact nucleus, which suggests that mitosis was not initiated in these eggs. A few eggs appeared a darker orange color, suggesting changes in the structure of pigments. Changes in LC3B processing were not observed either in western blots or after immunocytochemistry labeling when leupeptin and pepstatin were added to eggs treated with or without U0126 and/or roscovitine (results not shown). These results indicate that leupeptin and pepstatin led to a marked increase in the survival of eggs treated with or without U0126 and/or roscovitine without inducing an autophagy pathway.
Figure 9. Leupeptin and pepstatin enhance survival of eggs. Light microscopy images of control eggs (C) or eggs treated with 20 µM leupeptin and 7 µM pepstatin (L), 5 µM U0126 (U), 5 µM U0126 and 20 µM roscovitine (UR), U0126 and leupeptin (UL) or with U0126, leupeptin and roscovitin (URL). Eggs were observed 24 h after addition of the drugs. Although all eggs had fragmented without leupeptin and pepstatin, these drugs led to a marked increase in the survival of eggs treated with or without U0126 and/or roscovitine. Note the presence of dark eggs (stars) in the presence of leupeptin and pepstatin.
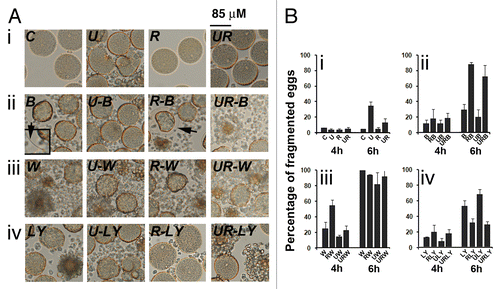
We then used bafilomycin A1 (Baf) that inhibits lysosomal acidification and blocks activity of the pH-dependent lysosomal proteases, thus leading to inhibition of autophagic fusion and accumulation of early autophagic vacuoles.Citation38 Eggs were treated with or without 2 µM U0126 and/or 20 µM roscovitin for 3 or 5 h and further treated with 100 nM Baf for one h. Morphology is shown in and results summarized in . As described above, fragmentation of eggs was induced after 6 h treatment with U0126 and was reduced in the presence of roscovitine (, i and B, i). Eggs treated with Baf only elevated a fertilization membrane, which indicates release in Cai (, ii). Baf only slightly increased the percentage of fragmentation in aging eggs, but dramatically stimulated cell death of eggs treated for 4 h with roscovitine with or without U0126, suggesting that no survival process took place in these eggs (, ii and B, ii).
Figure 10. Bafilomycin A1 (Baf) and PtdIns 3-kinase inhibitors act on egg survival. (A) Light microscopy images of control eggs (C) and of eggs treated for 6 h with U0126 (U), roscovitine (R), Baf (B), wortmannin (W), LY294002 (LY), or different combinations of these inhibitors. (B) Assessment of fragmentation percentages measured after 4 h and 6 h treatment. Effect of U0126 and roscovitine treatment (i), Baf (ii), wortmannin (iii) or LY294002 (iv) addition. Activation of the eggs with elevation of a fertilization envelope (inset, 2× magnification) can be seen in Baf-treated eggs (ii).
Finally, we investigated whether PtdIns 3-kinase played a role in the cell death programs triggered in unfertilized eggs.Citation39 We used wortmannin and LY294002, two PtdIns 3-kinase inhibitors that block the production of PtdIns3P which is essential for the initiation of autophagy.Citation40 Addition of 20 µM wortmannin powerfully induced fragmentation in eggs treated with or without U0126 and/or roscovitine (, iii and B, iii). Similar results were obtained with 20 µM LY294002 although the effect of this inhibitor seemed to be less drastic than that obtained with wortmannin (, iv and B, iv). These results suggest that PtdIns 3-kinase is required for survival in unfertilized eggs, even in conditions where no sign of autophagy is detected, i.e., in naturally aging eggs or eggs induced to die by apoptosis after inactivation of MAPK1/3.
Discussion
Our results suggest that unfertilized sea urchin eggs contain different routes capable of directing them toward cell death. This fits with the diversified families of proteins involved in cell death pathways expressed in this species.Citation41
Role of a high MAPK1/3 activity in unfertilized eggs
The main role of the active MAP2K-MAPK1/3 cascade in G1-arrested sea urchin eggs is to block a cell cycle oscillator relying on CDKs, as reported in almost all eumetozoan oocytes studied to date, no matter the stage of the cell cycle arrest, metaphase or interphase.Citation8,Citation15-Citation18,Citation20 As found in starfish and ascidian eggs, MAPK1/3 inactivation in sea urchin eggs triggers successive cycles of NEB/NE reformation that were mirrored by oscillations in P-CDK1(Tyr15) and were cancelled by roscovitine. The repetitive constrictions-relaxations that occurred with a similar period may be due either to surface contraction waves associated with MPF activation and inactivationCitation42 or to failed cleavage attempts, since such eggs lack sperm-derived centrosomes.
Apoptosis
This type of cell death would be the consequence of an aberrant entry into mitosis followed by a short attempt of proliferation that occurs after a rapid decline of MAPK1/3 activity, as it was significantly delayed by roscovitine or by injection of EGTA buffer, which inhibits MPF activationCitation43 and NEB in sea urchin zygotes.Citation26,Citation31 However, we cannot rule out the possibility that inactivation of MAPK1/3 stimulated cell death independently of mitosis entry, for example by acting directly on the phosphorylation of the two proapoptotic proteins BCL2L11/Bim and BAD.Citation44 Activation of CASP3 occurring well after the beginning of mitotic cycles and formation of huge blebs of the plasma membrane in dying eggs are characteristics of apoptosis. However, we were not able to detect DNA fragmentation, which is another feature of apoptosis and DNA degradation could only be observed at very final steps of cytoplasmic disintegration (not shown). Such difficulty to assess DNA fragmentation has been reported by others.Citation10 Lack of nuclear fragmentation is one of the features of necrosis, a caspase-independent mechanism,Citation6 which can then be ruled out here. “Mitotic catastrophe,” which occurs either during or shortly after a deregulated/failed mitosis culminating in apoptosis,Citation45 could take place in unfertilized eggs deprived of P-MAPK1/3 activity. These results contradict those obtained in starfish eggs where apoptosis occurring in aging mature eggs is delayed by inactivation of MAPK1/3 despite a similar entry into mitotic cell cycles.Citation15,Citation20,Citation21 Mature G1-arrested sea urchin eggs, that can be kept for days before fertilization, and starfish eggs that die 12 to 14 h after completion of meiosis, might use different survival mechanisms.
Cai changes mediate different cell death cascades.Citation27,Citation46 Here, they facilitated cell death induced by U0126, but it is difficult to determine whether they promoted mitotic cycling or acted directly on the apoptotic cascade. The first Cai increases induced by U0126 appear before any sign of CASP3 activation or fragmentation, suggesting that they are part of the mitotic oscillator. Finally inhibition of M phase entry by preventing Cai changes may have delayed cell death by driving the egg toward autophagy as did roscovitine. However, the low number of EGTA injected eggs made it difficult to test this theory.
Autophagy
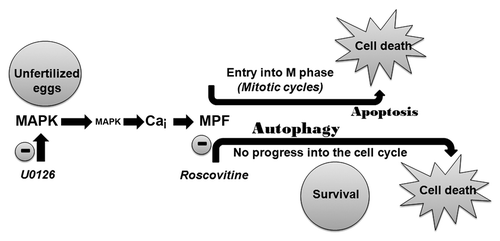
Autophagy would be used by unfertilized eggs as a protective mechanism to resist a cell death program triggered after MAPK1/3 inactivation when CDKs are also inhibited (). Cleavage of LC3B, increase in the particulate distribution and association of this protein to vesicular structures, observed under EM, corroborate this hypothesis. Structures that resemble autophagic compartmentsCitation36 were never seen in control eggs or eggs treated with U0126 alone, suggesting that their accumulation was due to increased formation of new autophagosomes and not to decreased clearance of normally formed autophagosomes/autolysosomes. Our results, showing the number of autophagic-like structures and of immunogold particles after LC3B staining, can then be considered as representative of an egg population as they were determined on several eggs that were cut at different levels of depth.Citation36 Moreover, these eggs contained a much dilated endoplasmic reticulum and an increase in AVOs was measured, which are also characteristic of autophagy.Citation34,Citation47,Citation48
Figure 11. Apoptosis or autophagy can be induced in the unfertilized egg after inactivation of MAPK1/3. A Ca and MPF-dependent pathway drives the egg through mitotic cycles that culminate in apoptosis. Inhibiting MPF delays cell death by diverting the egg through an autophagy program.
The rapid fragmentation induced in these eggs after injection of the anti-LC3B antibody reinforces the role of LC3B during survival. However, we found that some of the assays that are currently used to monitor autophagyCitation32 either could not be performed in the sea urchin egg or led to results that may be due to events that are not necessarily related to this process.Citation49,Citation50 Baf triggered a massive and rapid fragmentation in these eggs, which could indeed suggest that Baf inhibited a survival program. However, Baf is a specific inhibitor of the vacuolar type H+-ATPase and can also induce an elevation of cytosolic Ca2+.Citation51 This corroborates the elevation of a fertilization membrane in unfertilized eggs, treated with or without U0126 and/or roscovitine in the presence of Baf. Sea urchin eggs and embryos contain a broad panel of acidic organelles besides lysosomes, including cortical granules, endosomes, pigment granules and acidocalsisomes.Citation52 Baf may thus also act in sea urchin eggs on Ca and/or pH dependent events that are not necessarily involved in an autophagic process. Therefore, we feel that results obtained with this drug and the monitoring of the behavior of acidic compartments to assess autophagy in this species should be taken with caution.
We also used a combination of leupeptin and pepstatin that are commonly used to assess the autophagy process.Citation37 These drugs greatly increased survival of eggs that kept an intact nucleus until death, suggesting that they did not enter mitosis, even after treatment with U0126. Therefore, inhibition of proteases may have also altered cell cycle progress. For example, leupeptin can inhibit cleavage of BID, a proapoptotic protein that also promotes progression into S phase.Citation53 Leupeptin did not delay cell death by acting on some mechanisms involved in autophagy since we did not observe in these eggs any modification in LC3B processing (results not shown). Results obtained with these drugs to monitor autophagy should therefore be used with caution. Finally, it is impossible to perform inactivation of autophagy-related genes (e.g., ATG7, BECN1)Citation6,Citation32 in unfertilized sea urchin eggs that are spawned after meiosis, at G1 of the first mitotic cycle.
Cell death in aging eggs
Our hypothesis was that roscovitine had delayed the start of a cell death program by preventing entry into mitosis. However, 30 h-long recordings using a video-microscope as performed in , i revealed that untreated eggs kept their nucleus until fragmentation occurred (not shown). This suggests that these naturally aging eggs never entered mitosis and that the mechanisms leading to egg fragmentation are different from those that occur during U0126-induced cell death.
It is likely that a rapid decrease in MAPK1/3 activity (ca. 30 min.) induced after treatment with U0126 leads to a different cell death pathway from that taking place during natural aging of unfertilized eggs where egg fragmentation occurs after a slow and moderate decline in MAPK1/3 activity without entering mitosis. However, roscovitine delayed egg fragmentation in untreated/aging eggs, which suggests that some CDK-dependent mechanisms are involved in fragmentation of these eggs. The progressive loss in MAPK1/3 activity may occur together with that of other enzymes or mechanisms that are necessary for a cell to live. Since neither necrosis nor autophagy signs are observed in aging eggs, and since these eggs showed huge blebs before fragmentation, we can hypothesize that aging eggs die via an apoptotic pathway.Citation54 Finally, our results suggest that the class III PtdIns 3-kinase is involved in the survival of eggs, whether deprived or not of MAPK1/3 and/or CDKs activities. This corroborates the idea that an active PtdIns 3-kinase keeps cells alive by blocking apoptotic pathways or by having a positive effect on multiple prosurvival components.Citation55
In conclusion, we think that the sea urchin egg is an invaluable model to study how the cell cycle and cell death pathways are connected. Such connections have been found in hippocampal neurons from humans that develop Alzheimer disease, and are induced to undergo cell death after aberrant entry into the cell cycle.Citation56
Materials and Methods
Handling of gametes
Sea urchins Paracentrotus lividus were collected in Marseille, Villefranche-sur-mer, Roscoff or Ile de Ré (France). Gametes were collected, prepared and fertilized either in natural sea water (NSW) or in artificial sea water (ASW, Reef Crystals Instant Ocean) as described in our previous papers.Citation22,Citation23,Citation25
Inhibitors
Inhibitors were dissolved as stock solutions in DMSO at concentrations as follows: U0126 (Cell Signaling, 9903, 10 mM, 2 µM final), roscovitine (Sigma, R7772, 5 mM, 20 µM final), leupeptin (Sigma, L2884, 10 mM, 20 µM final), pepstatin (Sigma, P4265, 1.45 mM, 7 µM final), wortmannin (Sigma, W1628, 4 mM, 20 µM final), LY294002 (Cell Signaling, 9901, 4 mM, 20 µM final), bafilomycin A1 (Baf) (Sigma, B1793, 50 µM, 100 nM final). Inhibitors were added in the external medium to give a final concentration as indicated, and eggs were left in these dilutions until the end of the experiment. The appropriate addition of DMSO to the egg culture was used as a control of all experiments.
Cell cycle events and Ca imaging
In order to follow Cai changes and morphological events induced after MAPK1/3 and/or MPF inactivation during continuous long period of time, control or treated eggs were injected with Fura-2 dextran (10 kDa, Invitrogen, F-3029) to give an intracellular concentration of around 20 µM. Briefly, to excite the Fura 2 in the eggs, light emitted by a 75 w Xenon lamp was filtered through 340 and 380 nm band-pass filters housed in a filter wheel (Lambda 10 to 12/Sutter). Emitted light passed through a 510 nm dichroic filter and was collected using a 20× 0.75 na objective lens. Images were projected onto a cooled CCD camera (Princeton, Roper Scientific)Citation57 every min for 10 to 15 h at 18°C. Appropriate images were extracted from image stacks and manipulated with the imageJ software version 1.43u (NIH). Eggs were also observed under discontinuous bright-field illumination on an inverted Zeiss Axioplan microscope (Zeiss) equipped with DIC 20× optics and images taken with a Princeton CCD camera (Princeton, Roper Scientific) every min for 10 to 15 h at 18°C. z series were acquired with the Metamorph softwareCitation58 Appropriate images were extracted from image stacks and time series were reconstructed with the imageJ software.
Discontinuous observations of sample of eggs were performed under a Nikon Eclipse TE300 microscope (Nikon) equipped with a ×20 plan Nikon objective. Eggs showing clear extrusions of cytoplasm were counted as fragmented eggs.
Western blot analysis
Preparation of egg samples, separation of proteins by SDS-PAGE, western blotting and dilutions of all antibodies were performed as previously describedCitation22,Citation23,Citation25 with the following antibodies: Phospho-MAPK1/3 (ERK) mouse Ab (9106), MAPK1/3 (ERK) mouse Ab (9102), Phospho-CDK1(Tyr15)/CDC2(Tyr15) rabbit Ab (4539), total CDK1/CDC2 rabbit Ab (9112) and LC3B rabbit Ab (2775) from Cell Signaling, and α-tubulin (TUBA) mouse Ab (Calbiochem, CP06). The total amount of MAPK1/3 and CDK1 was detected after stripping and reblotting with the anti-MAPK1/3 and anti-CDK1 antibodies. Bands were analyzed using ImageJ. The ratios of P-MAPK1/3/MAPK1/3 and P-CDK1(Tyr15)/CDK1 were calculated and are given relative to the ratios measured at time zero and arbitrarily taken as 1. Bands detected with the anti-LC3 Ab (LC3B-I and LC3B-II) were normalized by comparison to TUBA (α-tubulin) and are expressed relative to the value determined in control eggs arbitrarily taken as 100.
CASP3 activity
A 5% egg suspension was treated with U0126 or Emetine. Five-hundred microliter samples were taken at different times before and after sperm or emetine addition and rapidly centrifuged. Egg pellets were immediately frozen and stored in liquid nitrogen until the kinase assay was performed. CASP3 activity was determined with a fluorimetric CaspACETM Assay System (Promega, G7351) on egg pellets that were defrosted in the assay buffer provided with the kit, according to the manufacturer's instructions.
LC3B labeling
Samples of eggs were taken before and at different times of treatment as indicated in the text, rinsed once within ASW-0Ca and fixed in a Fixation Buffer (FB2: 800 mM glycerol, 20 mM PIPES, 5 mM EGTA, 10 mM MgSO4, 0,1% Triton, 3.7% formaldehyde, 20 mM β-glycerophosphate, 2 mM sodium orthovanadate and 4 mM NaF. Eggs were then rinsed in TBS-2% Triton (TBS-T), and labeling performed as described previouslyCitation23 by using the anti-LC3B Ab and a goat anti-rabbit FITC Ab (Jackson), both diluted 1/1000 in TBS-T. At the end of the labeling procedure, eggs were stuck on a polylysine coated slide, observed by epifluorescence with a Nikon Eclipse TE300 equipped with a 20× plan fluor Nikon objective, using FITC or rhodamine Exc/Em filters, and transmitted light images were taken. Confocal analysis was then performed as follows: Green fluorescence was detected by confocal microscopy with a Zeiss LSM 700 microscope (Zeiss), equipped with a 488-nm laser and a 40x objective. A stack of 30 images taken every 3 µm was acquired, thus giving the entire depth of eggs that are approximately 85 µm in diameter. The number of large LC3B structures was quantified by ImageJ as follows. All 8-bit images of the stacks were binarized after automatic threshold calculation. Particles larger than 30 pixels2 (2.95 µm2) contained in the entire egg surface were counted in each confocal section. The total amount of particles for the stack of images was then summarized, thus giving an estimation of the total amount of LC3B structures/egg.
Acridine orange (AO) labeling
A sample of egg population was taken 12 h after incubation with U0126 and/or roscovitine, incubated for 20 min with 5 µg/ml AO (5 mg/ml stock solution), and then rinsed 3 times with ASW. Green or red fluorescence was detected by confocal microscopy as explained above for LC3B, by using 488 nm and 555 nm lasers and a 20× objective. An estimation of the total amount per egg of AO vesicles (AVO) that were either smaller (from zero) or larger than 30 pixels2 (2.95 µm2) was obtained as explained above. The global formation of AVO/egg is also given as the ratio of the red and green fluorescence intensities of total egg areas visualized by epifluorescence and quantified by ImageJ.
Electron microscopy observations
For identification of autophagic compartments by morphology, eggs were treated as follows: Before and at different times during treatment, as indicated in the text, samples of eggs were taken and rinsed once with in ASW-0Ca (490 mM NaCl, 27 mM MgCl2, 27 mM MgSO4, 10 mM KCl, 2 mM Na2HCO3, 1 mM EGTA, pH 8.0) and then incubated for 1 h in a Fixation Buffer (FB: 200 mM Na cacodylate, 200 mM NaCl, 0.35% glycerol, 1 mM EGTA, 1 mM MgSO4, 10 mM KCl) containing 4% paraformaldehyde, 0.1% glutaraldehyde and 0.12% picric acid. Eggs were rinsed once with FB and then incubated 30 min with FB containing 1% osmium. After a 30 min rinse in distilled water, eggs were dehydrated in successive ethanol dilutions (30, 50, and 70%), left overnight in ethanol 70 °C at 4 °C, and then dehydrated in ethanol 90% by 3 successive 1 h rinses. They were finally included in LR White resin (Media Medium, Sigma). Observations were performed under a Philips Transmission Electron Microscope (TEM 208, Philips Electron Optics) with a Hamamatsu CCD camera AMT. For an ultrastructure study, ultrathin sections were made (70 nm), disposed on Ni 200 mesh formvar/carbon grids. Sections were contrasted with 0.5% uranyl acetate for 15 min and 3% lead citrate for 5 min. For an immunolabeling study, ultrathin sections were made (50 nm), disposed on Ni 200mesh formvar/carbon grids. Grids were placed for 15 min in a PBS buffer (50 mM KH2PO4, 100 mM NaCl, pH 7.2) containing 50 mM glycine, once for 30 min and twice for 5 min in PBS containing 1% BSA (PBS-BSA). Grids were then incubated with the anti-LC3B antibody (Cell signaling, 1/30 in PBS-BSA) overnight at 4°C, rinsed 4 times for 5 min in PBS-BSA, incubated 45 min with the secondary antibody (Auroprobe goat anti-rabbit gold labeling 5 nm, Amersham, 1/30 dilution in PBS-BSA) and rinsed 3 times for 5 min in PBS-BSA and 3 times for 5 min in distilled water. They were contrasted with uranyl acetate 5% for 4 min and rinsed 3 times for 5 min in distilled water.
| Abbreviations: | ||
| AO | = | acridine orange |
| AVOs | = | acidic vesicular organelles |
| ASW | = | artificial sea water |
| Baf | = | bafilomycin A1 |
| BCL2L11 | = | BCL2-like 11 (apoptosis facilitator) |
| BAD | = | BCL2-associated agonist of cell death |
| BECN1 | = | Beclin 1, autophagy related |
| Cai | = | free cytosolic calcium concentration |
| CASP3 | = | caspase 3, apoptosis-related cysteine peptidase |
| CDK | = | cyclin-dependent kinase |
| EGTA | = | ethylene glycol tetraacetic acid |
| EM | = | electron microscopy |
| ER | = | endoplasmic reticulum |
| LC3B | = | microtubule-associated protein 1 light chain 3 beta |
| MAPK1/3 | = | mitogen-activated protein kinase 1/3 |
| Mos | = | Moloney sarcoma oncogene |
| MPF | = | maturation-promoting factor |
| NSW | = | natural sea water |
| NEB | = | nuclear envelope breakdown |
| TUBA | = | α-tubulin |
Additional material
Download Zip (9 MB)Acknowledgments
We thank R Dumollard and A Md Dougall for their help in Ca measurements, S Guyon for her help in confocal microscopy analysis and C Billam for correcting our manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/autophagy/article/25712
References
- Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod 2002; 66:1828 - 37; http://dx.doi.org/10.1095/biolreprod66.6.1828; PMID: 12021069
- Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction 2002; 124:745 - 54; http://dx.doi.org/10.1530/rep.0.1240745; PMID: 12530912
- Greenwood J, Gautier J. From oogenesis through gastrulation: developmental regulation of apoptosis. Semin Cell Dev Biol 2005; 16:215 - 24; http://dx.doi.org/10.1016/j.semcdb.2004.12.002; PMID: 15797832
- Lera S, Pellegrini D. Evaluation of the fertilization capability of Paracentrotus lividus sea urchin storaged gametes by the exposure to different aqueous matrices. Environ Monit Assess 2006; 119:1 - 13; http://dx.doi.org/10.1007/s10661-005-9000-0; PMID: 16767501
- Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 2009; 16:1093 - 107; http://dx.doi.org/10.1038/cdd.2009.44; PMID: 19373242
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al, Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009; 16:3 - 11; http://dx.doi.org/10.1038/cdd.2008.150; PMID: 18846107
- De Felici M, Lobascio AM, Klinger FG. Cell death in fetal oocytes: many players for multiple pathways. Autophagy 2008; 4:240 - 2; PMID: 18094606
- Oita E, Harada K, Chiba K. Degradation of polyubiquitinated cyclin B is blocked by the MAPK pathway at the metaphase I arrest in starfish oocytes. J Biol Chem 2004; 279:18633 - 40; http://dx.doi.org/10.1074/jbc.M311122200; PMID: 14985367
- Sasaki K, Chiba K. Fertilization blocks apoptosis of starfish eggs by inactivation of the MAP kinase pathway. Dev Biol 2001; 237:18 - 28; http://dx.doi.org/10.1006/dbio.2001.0337; PMID: 11518502
- Voronina E, Wessel GM. Apoptosis in sea urchin oocytes, eggs, and early embryos. Mol Reprod Dev 2001; 60:553 - 61; http://dx.doi.org/10.1002/mrd.1120; PMID: 11746966
- Nezis IP, Papassideri I. Monitoring autophagy in insect eggs. Methods Enzymol 2008; 451:669 - 83; http://dx.doi.org/10.1016/S0076-6879(08)03237-0; PMID: 19185745
- Chiarelli R, Agnello M, Roccheri MC. Sea urchin embryos as a model system for studying autophagy induced by cadmium stress. Autophagy 2011; 7:1028 - 34; http://dx.doi.org/10.4161/auto.7.9.16450; PMID: 21628995
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 2007; 1773:1213 - 26; http://dx.doi.org/10.1016/j.bbamcr.2006.10.005; PMID: 17112607
- Kishimoto T. Cell-cycle control during meiotic maturation. Curr Opin Cell Biol 2003; 15:654 - 63; http://dx.doi.org/10.1016/j.ceb.2003.10.010; PMID: 14644189
- Hara M, Mori M, Wada T, Tachibana K, Kishimoto T. Start of the embryonic cell cycle is dually locked in unfertilized starfish eggs. Development 2009; 136:1687 - 96; http://dx.doi.org/10.1242/dev.035261; PMID: 19369392
- Kondoh E, Tachibana K, Deguchi R. Intracellular Ca2+ increase induces post-fertilization events via MAP kinase dephosphorylation in eggs of the hydrozoan jellyfish Cladonema pacificum. Dev Biol 2006; 293:228 - 41; http://dx.doi.org/10.1016/j.ydbio.2006.02.005; PMID: 16530749
- Amiel A, Leclère L, Robert L, Chevalier S, Houliston E. Conserved functions for Mos in eumetazoan oocyte maturation revealed by studies in a cnidarian. Curr Biol 2009; 19:305 - 11; http://dx.doi.org/10.1016/j.cub.2008.12.054; PMID: 19230670
- Dumollard R, Levasseur M, Hebras C, Huitorel P, Carroll M, Chambon JP, McDougall A. Mos limits the number of meiotic divisions in urochordate eggs. Development 2011; 138:885 - 95; http://dx.doi.org/10.1242/dev.057133; PMID: 21303846
- Wu JQ, Kornbluth S. Across the meiotic divide - CSF activity in the post-Emi2/XErp1 era. J Cell Sci 2008; 121:3509 - 14; http://dx.doi.org/10.1242/jcs.036855; PMID: 18946022
- Tachibana K, Tanaka D, Isobe T, Kishimoto T. c-Mos forces the mitotic cell cycle to undergo meiosis II to produce haploid gametes. Proc Natl Acad Sci U S A 2000; 97:14301 - 6; http://dx.doi.org/10.1073/pnas.97.26.14301; PMID: 11121036
- Sasaki K, Chiba K. Induction of apoptosis in starfish eggs requires spontaneous inactivation of MAPK (extracellular signal-regulated kinase) followed by activation of p38MAPK. Mol Biol Cell 2004; 15:1387 - 96; http://dx.doi.org/10.1091/mbc.E03-06-0367; PMID: 14699071
- Chiri S, De Nadai C, Ciapa B. Evidence for MAP kinase activation during mitotic division. J Cell Sci 1998; 111:2519 - 27; PMID: 9701551
- Zhang WL, Huitorel P, Glass R, Fernandez-Serra M, Arnone MI, Chiri S, Picard A, Ciapa B. A MAPK pathway is involved in the control of mitosis after fertilization of the sea urchin egg. Dev Biol 2005; 282:192 - 206; http://dx.doi.org/10.1016/j.ydbio.2005.03.008; PMID: 15936340
- Philipova R, Kisielewska J, Lu P, Larman M, Huang JY, Whitaker M. ERK1 activation is required for S-phase onset and cell cycle progression after fertilization in sea urchin embryos. Development 2005; 132:579 - 89; http://dx.doi.org/10.1242/dev.01607; PMID: 15634691
- Zhang WL, Huitorel P, Geneviere AM, Chiri S, Ciapa B. Inactivation of MAPK in mature oocytes triggers progression into mitosis via a Ca2+ -dependent pathway but without completion of S phase. J Cell Sci 2006; 119:3491 - 501; http://dx.doi.org/10.1242/jcs.03082; PMID: 16912079
- Whitaker M. Calcium microdomains and cell cycle control. Cell Calcium 2006; 40:585 - 92; http://dx.doi.org/10.1016/j.ceca.2006.08.018; PMID: 17045645
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008; 27:6407 - 18; http://dx.doi.org/10.1038/onc.2008.308; PMID: 18955969
- Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 2010; 18:533 - 43; http://dx.doi.org/10.1016/j.devcel.2010.02.013; PMID: 20412769
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997; 243:527 - 36; http://dx.doi.org/10.1111/j.1432-1033.1997.t01-2-00527.x; PMID: 9030781
- Philipova R, Larman MG, Leckie CP, Harrison PK, Groigno L, Whitaker M. Inhibiting MAP kinase activity prevents calcium transients and mitosis entry in early sea urchin embryos. J Biol Chem 2005; 280:24957 - 67; http://dx.doi.org/10.1074/jbc.M414437200; PMID: 15843380
- Wilding M, Wright EM, Patel R, Ellis-Davies G, Whitaker M. Local perinuclear calcium signals associated with mitosis-entry in early sea urchin embryos. J Cell Biol 1996; 135:191 - 9; http://dx.doi.org/10.1083/jcb.135.1.191; PMID: 8858173
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008; 4:151 - 75; PMID: 18188003
- Kimura S, Fujita N, Noda T, Yoshimori T. Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Methods Enzymol 2009; 452:1 - 12; http://dx.doi.org/10.1016/S0076-6879(08)03601-X; PMID: 19200872
- Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ 2004; 11:448 - 57; http://dx.doi.org/10.1038/sj.cdd.4401359; PMID: 14713959
- Zakeri Z, Melendez A, Lockshin RA. Detection of autophagy in cell death. Methods Enzymol 2008; 442:289 - 306; http://dx.doi.org/10.1016/S0076-6879(08)01415-8; PMID: 18662576
- Ylä-Anttila P, Vihinen H, Jokitalo E, Eskelinen E-L. Monitoring autophagy by electron microscopy in Mammalian cells. Methods Enzymol 2009; 452:143 - 64; http://dx.doi.org/10.1016/S0076-6879(08)03610-0; PMID: 19200881
- Haspel J, Shaik RS, Ifedigbo E, Nakahira K, Dolinay T, Englert JA, Choi AM. Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy 2011; 7:629 - 42; http://dx.doi.org/10.4161/auto.7.6.15100; PMID: 21460622
- Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes?. Autophagy 2008; 4:849 - 950; PMID: 18758232
- Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 2008; 410:1 - 17; http://dx.doi.org/10.1042/BJ20071427; PMID: 18215151
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285:10850 - 61; http://dx.doi.org/10.1074/jbc.M109.080796; PMID: 20123989
- Robertson AJ, Croce J, Carbonneau S, Voronina E, Miranda E, McClay DR, Coffman JA. The genomic underpinnings of apoptosis in Strongylocentrotus purpuratus.. Dev Biol 2006; 300:321 - 34; http://dx.doi.org/10.1016/j.ydbio.2006.08.053; PMID: 17010332
- Rankin S, Kirschner MW. The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr Biol 1997; 7:451 - 4; http://dx.doi.org/10.1016/S0960-9822(06)00192-8; PMID: 9197242
- Beckhelling C, Penny C, Clyde S, Ford C. Timing of calcium and protein synthesis requirements for the first mitotic cell cycle in fertilised Xenopus eggs. J Cell Sci 1999; 112:3975 - 84; PMID: 10547358
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer?. Cell Cycle 2009; 8:1168 - 75; http://dx.doi.org/10.4161/cc.8.8.8147; PMID: 19282669
- Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ 2008; 15:1153 - 62; http://dx.doi.org/10.1038/cdd.2008.47; PMID: 18404154
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ 2007; 14:1267 - 74; http://dx.doi.org/10.1038/sj.cdd.4402147; PMID: 17431419
- Chakrabarti L, Eng J, Ivanov N, Garden GA, La Spada AR. Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol Brain 2009; 2:24; http://dx.doi.org/10.1186/1756-6606-2-24; PMID: 19640278
- Rodríguez-Muela N, Germain F, Mariño G, Fitze PS, Boya P. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ 2012; 19:162 - 9; http://dx.doi.org/10.1038/cdd.2011.88; PMID: 21701497
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009; 43:67 - 93; http://dx.doi.org/10.1146/annurev-genet-102808-114910; PMID: 19653858
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010; 22:124 - 31; http://dx.doi.org/10.1016/j.ceb.2009.11.014; PMID: 20034776
- Zhdanov AV, Dmitriev RI, Papkovsky DB. Bafilomycin A1 activates respiration of neuronal cells via uncoupling associated with flickering depolarization of mitochondria. Cell Mol Life Sci 2011; 68:903 - 17; http://dx.doi.org/10.1007/s00018-010-0502-8; PMID: 20820851
- Morgan AJ. Sea urchin eggs in the acid reign. Cell Calcium 2011; 50:147 - 56; http://dx.doi.org/10.1016/j.ceca.2010.12.007; PMID: 21251713
- Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene 2006; 369:7 - 19; http://dx.doi.org/10.1016/j.gene.2005.10.038; PMID: 16446060
- Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol 2008; 9:378 - 90; http://dx.doi.org/10.1038/nrm2393; PMID: 18414491
- Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J 2008; 415:333 - 44; http://dx.doi.org/10.1042/BJ20081056; PMID: 18842113
- Yang Y, Herrup K. Cell division in the CNS: protective response or lethal event in post-mitotic neurons?. Biochim Biophys Acta 2007; 1772:457 - 66; http://dx.doi.org/10.1016/j.bbadis.2006.10.002; PMID: 17158035
- Dumollard R, Hammar K, Porterfield M, Smith PJ, Cibert C, Rouvière C, Sardet C. Mitochondrial respiration and Ca2+ waves are linked during fertilization and meiosis completion. Development 2003; 130:683 - 92; http://dx.doi.org/10.1242/dev.00296; PMID: 12505999
- Prodon F, Chenevert J, Hébras C, Dumollard R, Faure E, Gonzalez-Garcia J, Nishida H, Sardet C, McDougall A. Dual mechanism controls asymmetric spindle position in ascidian germ cell precursors. Development 2010; 137:2011 - 21; http://dx.doi.org/10.1242/dev.047845; PMID: 20463032