Abstract
We recently reported that BAG6/BAT3 (BCL2-associated athanogene 6) is essential for basal and starvation-induced autophagy in E18.5 bag6−/− mouse embryos and in mouse embryonic fibroblasts (MEFs) through the modulation of the EP300/p300-dependent acetylation of TRP53 and autophagy-related (ATG) proteins. We observed that BAG6 increases TRP53 acetylation during starvation and pro-autophagic TRP53-target gene expression. BAG6 also decreases the EP300 dependent-acetylation of ATG5, ATG7, and LC3-I, posttranslational modifications that inhibit autophagy. In addition, in the absence of BAG6 or when using a mutant of BAG6 exclusively located in the cytoplasm, autophagy is inhibited, ATG7 is hyperacetylated, TRP53 acetylation is abrogated, and EP300 accumulates in the cytoplasm indicating that BAG6 is involved in the regulation of the nuclear localization of EP300. We also reported that the interaction between BAG6 and EP300 occurs in the cytoplasm rather than the nucleus. Moreover, during starvation, EP300 is transported to the nucleus in a BAG6-dependent manner. We concluded that BAG6 regulates autophagy by controlling the localization of EP300 and its accessibility to nuclear (TRP53) and cytoplasmic (ATGs) substrates.
The role of the acetylation of key proteins involved in autophagy has been highlighted by recent studies. In mammalian cells, ULK1 acetylation by the acetyltransferase KAT5/TIP60 is essential for the stimulation of autophagy after growth-factor deprivation whereas the acetylation of ATG5, ATG7, ATG12, or LC3 by the acetyltransferase EP300 inhibits autophagy. In addition to ATG proteins, acetylation also targets other critical proteins. Our recent work demonstrates that TRP53 acetylation on lysine 373 by EP300 is essential for autophagy. Surprisingly, the role of EP300 in autophagy seems contradictory because, in the cytosol, the EP300-dependent acetylation of ATG proteins inhibits autophagy, whereas it stimulates autophagy through the nuclear EP300-dependent acetylation of TRP53.
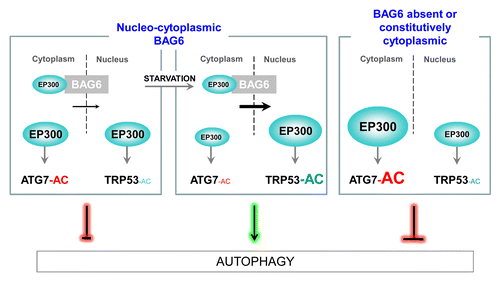
We found that the protein BAG6 tightly modulates autophagy through the regulation of the intracellular localization of EP300 (). BAG6 is a 150-kDa nucleocytoplasmic protein known, among others, for its role in apoptosis and cytoplasmic quality control. BAG6 contains many structural domains including a nuclear export signal and a nuclear localization signal. First, we demonstrated that BAG6 is essential for basal autophagy in mice embryos (E18.5) and for basal and starvation-induced autophagy in wild-type (WT) and bag6−/− MEFs. Indeed, the absence of BAG6 reduces the number of autophagosomes per cell and the autophagic flux, showing that BAG6 is essential for autophagosome formation. We next demonstrated the critical role of BAG6 in the EP300-dependent acetylation of TRP53 during starvation leading to the upregulation of the pro-autophagic TRP53-target gene Sesn1/Sestrin 1. Controversially, we noticed that BAG6 decreases the level of acetylation of ATG5, ATG7, and LC3-I. EP300 is a nuclear protein. Interestingly, we observed a strong accumulation of EP300 in the cytosol in the absence of BAG6, which correlates with an inhibition of TRP53 acetylation and a stimulation of ATG protein acetylation. Similar results are obtained when using a mutant of BAG6 lacking the nuclear localization signal, which constitutively localizes in the cytoplasm. In fact, when BAG6 is constitutively cytoplasmic, ATG7 is hyperacetylated, TRP53 acetylation is not increased during starvation, and autophagy is abrogated. In WT MEFs, we observed a strong interaction between BAG6 and EP300 in the cytoplasm compared with the nucleus. Moreover, during starvation we showed that EP300 is relocated from the cytosol to the nucleus only if BAG6 is present. Based on these results, we propose that BAG6 is involved in the nuclear localization of EP300, a process that occurs in basal conditions and that is increased during starvation. Thus, BAG6 is in charge of a small pool of the acetyltransferase EP300 that shuttles between the nucleus and the cytosol, then regulating autophagy when needed by modifying the access of EP300 to its substrates.
Figure 1. BAG6 inhibits EP300-dependent acetylation (AC) of ATG7 but stimulates the nuclear acetylation of TRP53 during starvation. In the cytosol, BAG6 inhibits ATG7 acetylation, an inhibitory event in autophagy, by limiting the quantity of the acetyltransferase EP300 available in the cytosol. On the contrary, by favoring the nuclear transport of EP300 under starvation conditions, BAG6 i) stimulates the nuclear TRP53 acetylation and ii) maintains a low level of ATG7 acetylation, both leading to the stimulation of autophagy. When BAG6 is absent or constitutively located into the cytoplasm, autophagy is inhibited. Concomitantly, a strong accumulation of cytoplasmic EP300 occurs, leading to the hyperacetylation of ATG7 and the inhibition of TRP53 acetylation upon starvation.
| Abbreviations: | ||
| ATG proteins | = | autophagy-related proteins |
| BAG6 | = | BCL2-associated athanogene 6 |
| MEFs | = | mouse embryonic fibroblasts |
| WT | = | wild-type |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by the Institut National de la Santé et de la Recherche Médicale (CG, PC, LKL, EL-C, and SP), by the ARC foundation (SP), La Ligue Régionale contre Le Cancer (Comités du Gard et de l’Hérault, SP), Cancéropole Grand Sud-Ouest (SP), and the CHEMORES consortium (Tumour Chemotherapy Resistance EU FP6 to EL-C). SS has fellowships from the Fond National de la Recherche Luxembourg (FNR Luxembourg) and La Ligue Nationale contre le Cancer.
