Abstract
We recently showed that phagophore biogenesis requires SNAREs. Our data indicate that the exocytic Q/t-SNAREs Sso1/2 and Sec9 are required for one of the earliest steps in autophagosome biogenesis, the homotypic fusion of Atg9-containing vesicles. We propose that this step precedes the formation of Atg9-containing tubulovesicular clusters (TVCs) that is a key step in perivacuolar, phagophore assembly. We also found that the endosomal Q/t-SNARE Tlg2 and the R/v-SNAREs Sec22 and Ykt6 interact with Sso1-Sec9, and are required for normal Atg9 trafficking. Thus, autophagosome biogenesis appears to involve multiple SNARE-mediated fusion events. These findings provide novel insights into the mechanism of autophagosome construction.
One of the most highly debated questions in the autophagy field concerns the source of the membrane used in autophagosome biogenesis. Intense work from several labs has now implicated multiple membrane sources that contribute to autophagosome formation including the endoplasmic reticulum, Golgi, mitochondria, and the plasma membrane. Interestingly, an obstruction in membrane supply from any one of these sources results in a major block in autophagosome biogenesis. Such an outcome is not expected if each donor membrane contributes directly to the phagophore. Furthermore, how can multiple membranes of presumably distinct composition be used to generate a single compartment? Thus, a pressing question concerns the precise step in autophagosome formation that membrane from each of these sources contributes to, and the mechanism that coordinates the assembly of vesicles from these disparate membrane sources into functional autophagosomes.
Our lab recently reported that the Golgi is a potential membrane source for autophagy through the identification of the involvement of two exocytic proteins, the Rab GTPase Sec4, and its GEF, Sec2, in autophagosome formation. Downstream of Sec4 and Sec2 are the Q/t-SNAREs Sso1/2 and Sec9 and the R/v-SNAREs Snc1/2 that bring about the docking and fusion of exocytic vesicles with the plasma membrane. Accordingly, we investigated whether the exocytic Q/t-SNAREs are also required for autophagy. Indeed, sso1Δ/2ts or sec9-4 mutants are defective in autophagosome formation. In order to understand how these exocytic SNAREs affect autophagosome biogenesis we examined the localization pattern of Atg9 in the mutant strains. It has previously been shown that Atg9 localizes to TVCs, also called Atg9 reservoirs, which are found close to the mitochondria. According to the current model of autophagosome formation in yeast, translocation of Atg9-containing reservoirs close to the vacuole followed by the recruitment of other Atg proteins gives rise to the autophagosome precursor called the phagophore assembly site (PAS). Fluorescence microscopy analysis reveals that in the sso1Δ/2ts and sec9–4 mutants, the anterograde movement of Atg9 to the PAS is highly reduced, suggesting a limitation in membrane flow in these mutants. To get a more in-depth picture of Atg9-containing clusters in the sso1Δ/2ts mutant, we performed an immuno-EM analysis. The ultrastructure of Atg9-containing membrane structures in the sso1Δ/2ts mutant at the non-permissive temperature revealed two interesting phenotypes: First, the absence of Atg9-containing tubules; and second, a mixed population of vesicles of at least two different sizes—large vesicles between 80–100 nm and small ones between 30–40 nm. Based on previous reports, it is likely that the 100-nm diameter vesicles are exocytic vesicles that accumulate in the absence of Sso1 and Sso2. Intriguingly, in the sso1Δ/2ts mutant Atg9 appears to be preferentially associated with the smaller vesicles suggesting that they may be the progenitors of Atg9 TVCs, and that Sso1 and Sso2 are required for the higher order organization of these Atg9-containing vesicles (). Consistent with the role of the exocytic SNAREs in the homotypic fusion of Atg9-containing vesicles, we were able to observe a colocalization between Atg9-3GFP and RFP-Sso1. However, we did not observe any colocalization between the PAS marker RFP-Ape1 and GFP-Sso1, indicating that these SNAREs play a role in membrane trafficking steps prior to PAS assembly.
Figure 1. The exocytic Q/t-SNAREs Sso1/2 and Sec9 mediate the homotypic fusion of post-Golgi, Atg9-containing vesicles into Atg9 tubulovesicular clusters. This fusion step represents one of the earliest steps in autophagosome formation. Atg23 and Atg27 form a complex with Atg9, and affect its anterograde movement. It is therefore likely that TVCs contain Atg9, Atg23 and Atg27. Subsequent to TVC formation, one or more TVCs move from peripheral cellular sites to a perivacuolar site to form the phagophore assembly site. Additional SNARE-mediated fusion events may be required for the elongation of the TVCs to give rise to autophagosomes. The Atg9-homotypic fusion event may require a complex between Sso1, Sec9, and the v-SNAREs Sec22 or Ytk6. The endocytic Q/t-SNARE Tlg2 also forms a complex with Sso1 and Sec9, and affects Atg9 anterograde transport.
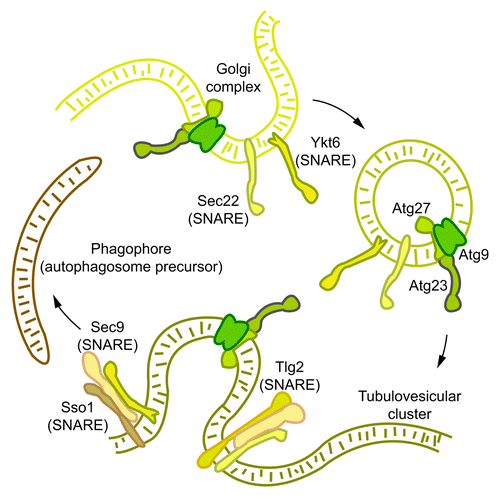
An examination of v-SNARE mutants revealed that the cognate R/v-SNAREs of Sso1/2, Snc1 and Snc2, are dispensable for autophagy. Therefore, we examined the role of other v-SNAREs, Nyv1 and Sec22, which have been shown to drive liposome fusion with Sso1 and Sec9, in autophagy. Although Nyv1 does not have a role in autophagy, two independent alleles of sec22 show severe blocks in autophagy. Similar to the sso1Δ/2ts mutant, both of the sec22 alleles also show defects in Atg9 anterograde transport. Additionally, we were also able to isolate a complex between Sso1, Sec9 and Sec22 after crosslinking, and observe some colocalization between Atg9 and Sec22. Unlike Sso1 and Sso2 that only have roles in Golgi-to-plasma membrane transport, Sec22 participates in ER-Golgi anterograde and retrograde transport. At this point, therefore, we do not know whether the defect in Atg9 anterograde transport occurs as a result of a defect in Atg9 trafficking through the ER in the sec22 mutants. Thus, it is important to determine whether the role of Sec22 in autophagosome formation is direct, and test by immuno-EM if this protein is required for Atg9 tubulovesicular clustering. Another R/v-SNARE that we tested for its role in autophagy is Ykt6, which participates in several membrane trafficking pathways and whose overexpression has previously been shown to rescue the growth and ER-Golgi trafficking defects of the sec22 temperature sensitive mutants at the nonpermisssive temperature. Ykt6 participates in homotypic vacuole fusion during which it has two separable functions; first, it may act as a tether for other SNAREs, and second, as an acetyltransferase of Vac8. Vac8 also has a role in autophagy, and therefore we need to determine which activity of Ykt6 is important for its autophagy function. In the current work, we also found that the endocytic Q/t-SNARE, Tlg2, that was previously shown to be required for the Cvt pathway, affects the magnitude of autophagy. The tlg2∆ mutant is also compromised in Atg9-anterograde transport, although this defect is not as severe as in the sso1Δ/2ts mutant. More detailed immuno-EM analysis is required in order to determine the role of this SNARE in phagophore assembly. Interestingly, while we were able to isolate a complex between Sso1, Sec9 and Tlg2, after crosslinking, this complex was unable to drive proteoliposome fusion in vitro. These results suggest at least three possibilities: First, even though we are able to isolate an Sso1-Sec9-Tlg2 complex by immunoprecipitation, such a complex does not drive fusion in vivo; second, the in vitro fusion requires some other factor(s); and third, Tlg2 needs to be modified post-translationally, as is the case with some SNAREs, in order to drive fusion.
In conclusion, we have found a role for SNAREs in a critical, early step in autophagosome biogenesis. Furthermore, the theme that is emerging regarding this process is the sharing of components that are required in other trafficking pathways for use in autophagosome formation. It will be exciting to decipher the precise roles of such components that our lab has shown to be required for autophagy thus far such as Ypt1, the COG complex, Sec2, Sec4 and most recently the SNARE proteins.