Abstract
We recently identified the atypical myosin, Myosin VI, as a component of epithelial cell-cell junctions that interacts with E-cadherin. Recombinant proteins bearing the cargo-binding domain of Myosin VI (Myo VI-CBD) or the cytoplasmic tail of E-cadherin can interact directly with one another. In this report we further investigate the molecular requirements of the interaction between Myo VI-CBD and E-cadherin combining truncation mutation analysis with in vitro binding assays. We report that a short (28 amino acid) juxtamembrane region of the cadherin cytoplasmic tail is sufficient to bind Myo VI-CBD. However, central regions of the cadherin tail adjacent to the juxtamembrane sequence also display binding activity for Myo VI-CBD. It is therefore possible that the cadherin tail bears two binding sites for Myosin VI, or an extended binding site that includes the juxtamembrane region. Nevertheless, our biochemical data highlight the capacity for the juxtamembrane region to interact with functionally-significant cytoplasmic proteins.
Introduction
Classical cadherin adhesion receptors mediate cell-cell interactions in all solid tissues in the body.Citation1-Citation3 This is exemplified by the prototypical epithelial cadherin, E-cadherin, which supports tissue morphogenesis during developmentCitation3 and whose function is often disrupted as carcinomas progress to invasion and metastasis.Citation4 E-cadherin, like other classical cadherins, functions as a membrane-spanning macromolecular complex. The cadherin ectodomain mediates adhesive binding while its cytoplasmic tail can interact, directly or indirectly, with a diverse range of cytoplasmic proteins that influence signaling, membrane trafficking and the cytoskeleton. Of the latter interacting proteins, the best understood are the catenins, which form a stoichiometric complex with cadherin.Citation5 Both β-catenin and p120-catenin bind directly to the cadherin cytoplasmic tail (),Citation6 while α-catenin is scaffolded into the cadherin complex by association with β-catenin.Citation7
It has long been recognized that cadherins function in close functional and biochemical cooperation with the actin cytoskeleton. Especial attention has focused on the role that α-catenin may play in allowing the cadherin molecular complex to bind actin filaments (reviewed in ref. Citation8). However, it is becoming apparent that cadherins can recruit, and associate, with a range of cytoskeletal proteins that regulate filament dynamics, organization and contractility. One such protein is the atypical myosin, Myosin VI, which localizes to cadherin junctions as they mature and contributes to that junctional development.Citation9 Myosin VI is unusual among myosins as it is a minus-end directed motor that is capable of functioning as a tension-activated anchor,Citation10,Citation11 linking cargo to the actin cytoskeleton. Co-immunoprecipitation analysis revealed that Myosin VI associates with E-cadherin and we recently reported that uncoupling of Myosin VI from E-cadherin contributed to acute junctional disruption when cells were treated with hepatocyte growth factor (HGF).Citation12 Myosin VI may then play an important role in regulated coupling of cadherins to the actin cytoskeleton. We further demonstrated the capacity for cadherin and Myosin VI to interact directly, as recombinant proteins bearing the C-terminal cargo-binding domain of Myosin VI could bind to the isolated cytoplasmic domain of E-cadherin.Citation12 In the current report we extend our in vitro analysis of the Myosin VI-cadherin interaction to further define the regions of these molecules responsible for their association.
Results
The Myosin VI tail contains several distinct regionsCitation10,Citation11 and we recently reported that the C-terminal cargo-binding domain (CBD) could directly interact with the cytoplasmic tail of E-cadherin in vitro.Citation12 This is demonstrated further in , showing the interaction of recombinant proteins consisting of the Myo VI-CBD bearing a Myc-tag and the cytoplasmic tail of E-cadherin that was fused to GST (E-cad-cyto).
Figure 2. C-terminal E-cadherin truncation mutants interact with the cargo-bindng domain of Myosin VI. Coomassie stained gel (A) and the corresponding Myc-immunoblot (B) of a representative in vitro binding experiment. The first two lanes show the E-cadherin tail construct coupled to GSH beads, without (lane 1) and with added myc tagged Myo VI CBD (lane 2). The following 6 lanes were loaded correspondingly with three truncated constructs (NSS, PYD and YYD), as outlined in . Lanes 9–12 are the negative GST controls and lane 13 indicates the molecular mass of the MyoVI CBD construct. The data are representative of three independent experiments.
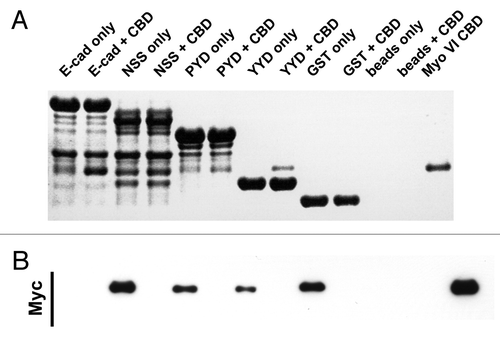
We then sought to further define the binding interaction between Myo VI-CBD and E-cad-cyto. Accordingly, we first constructed a series of c-terminal truncation mutants of the cadherin cytoplasmic domain (). These included E-Cad-cyto-NSS, which truncates within the β-catenin-binding domain; E-cad-cyto-PYD, which deletes the whole β-catenin-binding domain; and E-cad-cyto-YYD, which retains the juxtamembrane 28 amino acids of the cytoplasmic tail but removes the p120-catenin binding site as well as the β-catenin-binding site. All these mutants, including the shortest, E-cad-cyto-YYD, were able to interact with Myo VI-CBD with similar efficiency (). Although on occasions the short E-Cad-cyto-YYD mutant appeared to bind more strongly than the longer fragments (as illustrated in ), this was not a consistent finding. This suggested that the juxtamembrane region of the cadherin cytoplasmic tail might be sufficient to bind Myo VI-CBD.
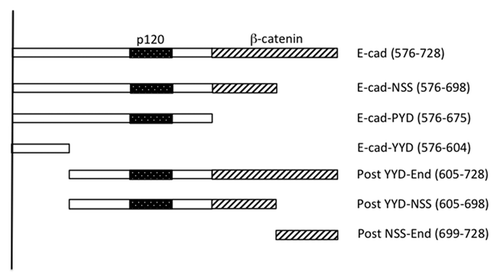
Figure 1. Schema of the E-cadherin cytoplasmic tail and mutants used in this study. The “core” p120 binding siteCitation13 and β-catenin binding site are identified. C-terminal truncations are named for the final (C-terminal) three amino acids in the truncation. The amino acid positions that mark the N- and C-termini of the fragments (numbered from the beginning of the mature protein) are indicated in brackets.
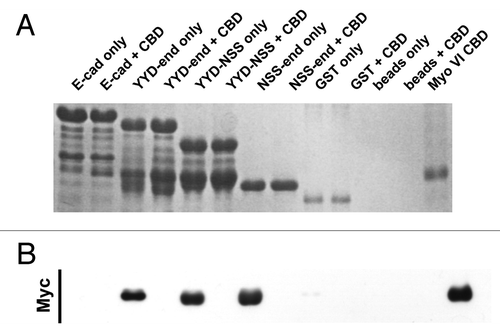
To extend our analysis, we constructed a further series of mutants that included N-terminal mutations of E-cad-cyto ( and ). A polypeptide bearing only the C-terminal 31 amino acids of the cytoplasmic tail did not bind Myo VI-CBD. However, two mutants that contain regions of the cytoplasmic tail adjacent to the juxtamembrane sequence also displayed the capacity to bind Myo VI-CBD. This suggests that the juxtamembrane sequence, although sufficient to bind Myo VI-CBD in vitro, may not be solely necessary for this interaction.
Figure 3. The juxtamembrane cadherin tail is not solely required to bind Myosin VI. Coomassie stained gel (A) and the corresponding Myc-immunoblot (B) of a representative in vitro binding experiment. The first two lanes show the E-cadherin tail construct coupled to GSH beads, without (lane 1) and with added myc tagged Myo VI CBD (lane 2). The following 6 lanes were loaded correspondingly with the three constructs (YYD-end, YYD-NSS and NSS-end) outlined in . Lanes 9–12 are the negative GST controls and lane 13 indicates the molecular mass of the Myo VI CBD construct. The data are representative of three independent experiments.
Finally, we sought to examine regions of the Myo VI-CBD that might be involved in its interaction with E-cadherin (). We were guided by the recently-solved structure of the association between Myo VI-CBD and Dab2,Citation14 which demonstrated that this interaction involves two separate binding sites in Myo VI-CBD. Although Dab2 and the cadherin cytoplasmic tail share no recognizable structural domains, they have some similar properties. Both the first CBD-binding domain of Dab2 and the juxtamembrane region of the cadherin cytoplasmic tail are enriched in hydrophobic residues. Further, the second binding site of Dab2 involves charge-charge and hydrophobic interactions in a “DHDDFD” motif; and, interestingly, a similar motif (DQDFD) is found in the E-cadherin cytoplasmic tail.
Figure 4. E-cadherin has binding requirements distinct from Dab2. Coomassie stained gel (A) and the corresponding Myc-immunoblot (B) of a representative in vitro binding experiment. The first two lanes show the E-cadherin tail construct coupled to GSH beads, without (lane 1) and with added myc tagged Myo VI CBD (lane 2). In lane 3 and 4 two point mutations of myosin VI (R1125E and L1209K) that inhibit the binding to Dab2 were tested in their ability to bind E-cad. Lanes 5–7 are negative GST controls and lane 8–10 show the three Myo VI CBD constructs loaded alone to indicate their expected molecular mass.
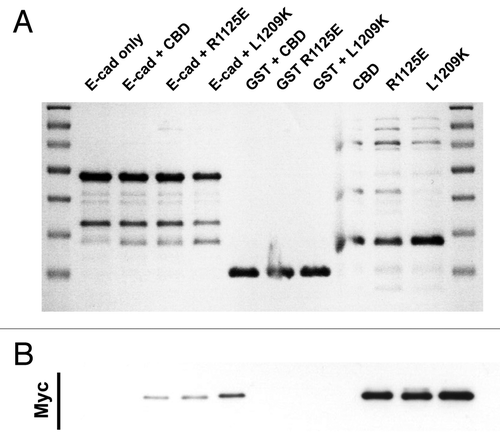
Accordingly, we introduced point mutations into site 1 (L1209K) and site 2 (R1152E) of the Myo VI-CBD that are reported to perturb its interaction with Dab2.Citation14 However, individually these mutations did not affect the ability of MyoVI-CBD to interact with the cadherin cytoplasmic tail (). We have not tested the double mutation of both sites to exclude redundancy in the binding mechanism, but the data suggests that cadherin binds to Myo VI by interactions distinct from those utilized by Dab2.
Discussion
These findings suggest that the juxtamembrane region of the cadherin cytoplasmic tail contributes to its interaction with Myosin VI. It is possible, however, that the cadherin cytoplasmic tail contains multiple binding sites for Myosin VI, as has been demonstrated for Dab2.Citation14 This is suggested by the observation that cadherin fragments lacking the juxtamembrane region could bind the Myosin VI CBD as well as the full-length cadherin tail (). In this model, one site may be the juxtamembrane domain, while others are found in the cytoplasmic tail adjacent to the juxtamembrane region. Alternatively, the Myosin VI binding site may involve an extended interface overlapping both the juxtamembrane domain and its adjacent region of the cytoplasmic tail. More detailed mutational and structural analysis will be necessary to resolve this issue.
Nevertheless, our data highlight the potential capacity of the juxtamembrane region to contribute to the cadherin molecular complex. It is generally acknowledged that their capacity to bind cytoplasmic proteins is central to the molecular mechanisms that allow classical cadherins to influence tissue organization. The best understood of these interactions include those with the “core” catenins (β-catenin, p120-catenin and α-catenin), which are well-established to form a stoichiometric complex. However, many other cytoplasmic proteins have been reported to interact with the cadherin cytoplasmic tail, generally by co-immunoprecipitation analysis.Citation1 These include a range of actin regulatory proteins and signaling molecules, many of which are likely to engage with the cadherin through interactions that are substoichiometric and much more transient than those of the core cadherin-catenin complex. Some of these interaction are indirect; for instance, centralspindlin recruits to cadherin junctions through an interaction with α-catenin.Citation15 However, other interactions are direct, as our data suggests is the case for Myosin VI.
Interestingly, the juxtamembrane region of the cytoplasmic tail has also been reported to associate with ankyrin,Citation16 an interaction that supported junctional integrity and may also influence the membrane traffic of E-cadherin. Further, the juxtamembrane region can make distinct contributions to cadherin function. One noteworthy example is the observation that a C-terminal cadherin mutant that deleted both the β- and p120-catenin binding sites, but retained a short juxtamembrane sequence comparable to that which we have now found to bind Myosin VI, served as an effective dominant-inhibitory mutant when expressed in Xenopus embryonic blastomeres.Citation17 It is interesting to speculate that these effects may have arisen through its capacity to sequester cytoskeletal proteins, such as ankyrin and Myosin VI. Together, these observations suggest that the juxtamembrane region of the cadherin cytoplasmic tail may contribute to cadherin function by mediating interactions with the actin cytoskeleton.
Materials and Methods
Molecular cloning
The human Myosin VI CBD was ligated into a modified pMWH6 protein expression vector which contains a 6-His and Myc tags as described previously.Citation12 The sequence for murine E-cad-cyto was cloned into pGEX-2T as previously described.Citation12 Truncation mutants of E Cad-cyto were generated by PCR amplification. The Myo VI-CBD L1209K mutant was generated by PCR mutagenesis using the QuikChange mutagenesis kit; the Myo VI-CBD R1152E mutant was synthesized by GeneArt.
Recombinant protein expression and in vitro binding assays
Recombinant proteins were expressed in E. coli, and protein binding studies performed, as previously reported.Citation12
Acknowledgments
We thank our laboratory colleagues for their support and many helpful discussions and Jim Spudich (Stanford) for the kind gift of Myosin VI plasmids. This work was supported by research grants from the National Health and Medical Research Council of Australia (631377) to ASY and from the Australian Research Council (DP0985029) to BMC. SM was supported by an ANZ Trustees PhD scholarship. ASY is an NHMRC Research Fellow (631383) and BMC an ARC Future Fellow (FT100100027).
References
- Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev 2011; 91:691 - 731; http://dx.doi.org/10.1152/physrev.00004.2010; PMID: 21527735
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 2006; 20:3199 - 214; http://dx.doi.org/10.1101/gad.1486806; PMID: 17158740
- Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol 2009; 89:33 - 54; http://dx.doi.org/10.1016/S0070-2153(09)89002-9; PMID: 19737641
- Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression?. Oncogene 2008; 27:6920 - 9; http://dx.doi.org/10.1038/onc.2008.343; PMID: 19029934
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A 1995; 92:5067 - 71; http://dx.doi.org/10.1073/pnas.92.11.5067; PMID: 7761449
- Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol 1995; 15:4819 - 24; PMID: 7651399
- Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol 1992; 116:989 - 96; http://dx.doi.org/10.1083/jcb.116.4.989; PMID: 1734027
- Maiden SL, Hardin J. The secret life of α-catenin: moonlighting in morphogenesis. J Cell Biol 2011; 195:543 - 52; http://dx.doi.org/10.1083/jcb.201103106; PMID: 22084304
- Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol 2007; 178:529 - 40; http://dx.doi.org/10.1083/jcb.200612042; PMID: 17664339
- Altman D, Sweeney HL, Spudich JA. The mechanism of myosin VI translocation and its load-induced anchoring. Cell 2004; 116:737 - 49; http://dx.doi.org/10.1016/S0092-8674(04)00211-9; PMID: 15006355
- Sweeney HL, Houdusse A. What can myosin VI do in cells?. Curr Opin Cell Biol 2007; 19:57 - 66; http://dx.doi.org/10.1016/j.ceb.2006.12.005; PMID: 17175153
- Mangold S, Wu SK, Norwood SJ, Collins BM, Hamilton NA, Thorn P, et al. Hepatocyte growth factor acutely perturbs actin filament anchorage at the epithelial zonula adherens. Curr Biol 2011; 21:503 - 7; http://dx.doi.org/10.1016/j.cub.2011.02.018; PMID: 21396819
- Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell 2010; 141:117 - 28; http://dx.doi.org/10.1016/j.cell.2010.01.017; PMID: 20371349
- Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, Zhao Y, et al. Myosin VI undergoes cargo-mediated dimerization. Cell 2009; 138:537 - 48; http://dx.doi.org/10.1016/j.cell.2009.05.030; PMID: 19665975
- Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, et al. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 2012; 14:818 - 28; http://dx.doi.org/10.1038/ncb2532; PMID: 22750944
- Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem 2007; 282:26552 - 61; http://dx.doi.org/10.1074/jbc.M703158200; PMID: 17620337
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell 1992; 69:225 - 36; http://dx.doi.org/10.1016/0092-8674(92)90404-Z; PMID: 1568244