Abstract
Recombinant glycoprotein drugs require proper glycosylation for optimal therapeutic efficacy. Glycoprotein therapeutics are rapidly removed from circulation and have reduced efficacy if they are poorly sialylated. Ricinus communis agglutinin-I (RCA-I) was found highly toxic to wild-type CHO-K1 cells and all the mutants that survived RCA-I treatment contained a dysfunctional N-acetylglucosaminyltransferase I (GnT I) gene. These mutants are named CHO-gmt4 cells. Interestingly, upon restoration of GnT I, the sialylation of a model glycoprotein, erythropoietin, produced in CHO-gmt4 cells was shown to be superior to that produced in wild-type CHO-K1 cells. This addendum summarizes the applicability of this cell line, from transient to stable expression of the recombinant protein, and from a lab scale to an industrial scale perfusion bioreactor. In addition, CHO-gmt4 cells can be used to produce glycoproteins with mannose-terminated N-glycans. Recombinant glucocerebrosidase produced by CHO-gmt4 cells will not require glycan remodeling and may be directly used to treat patients with Gaucher disease. CHO-gmt4 cells can also be used to produce other glycoprotein therapeutics which target cells expressing mannose receptors.
Introduction
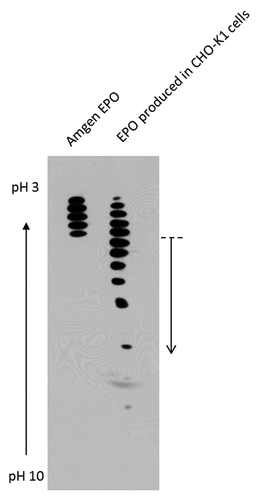
Sialylation of glycoprotein therapeutics plays an important role in regulating the circulatory half-life of recombinant drugs. Poorly-sialylated glycoproteins with exposed galactose residues bind to asialoglycoprotein receptors expressed on liver cells which leads to its removal from circulation, resulting in a shorter in vivo half-life of the therapeutic protein.Citation1,Citation2 This has been demonstrated in several recombinant therapeutic glycoproteins such as erythropoietin (EPO),Citation3 Factor VIII,Citation4 and chorionic gonadotrophin.Citation5 Commercially available recombinant EPO used to treat patients with anemia contains only highly sialylated isoforms as shown in the left lane of . However, transiently expressed recombinant EPO produced in CHO-K1 cells also contains many isoforms that are less sialylated as shown in the right lane in . The less sialylated isoforms, sometimes constituting up to 80% of the total product, has to be discarded during purification.Citation6 Hence, the biotechnology industry has focused on improving the sialylation of recombinant glycoproteins through host cell line engineering, process control and optimization.Citation7 We have developed a novel technology that is able to produce superiorly sialylated glycoprotein therapeutics with the use of novel glycosylation mutants, CHO-gmt4 cells.Citation8,Citation9
Figure 1. Isoelectric focusing and/or immunoblotting (IEF) illustrating the sialylation profile of commercial Amgen EPO (left) and transiently expressed EPO produced in CHO-K1 cells (right). EPO isoforms with better sialylation are found at the upper portion of the blot. The dotted line and arrow denotes the isoforms that would be discarded during purification.
The Isolation of CHO-gmt4 Cells that Possess Dysfunctional GnT I
CHO-gmt4 cells belong to a panel of CHO glycosylation mutant (CHO-gmt) lines that have been established in our group. All CHO-gmt lines are able to grow in suspension culture using chemically defined media to high cell densities; hence they are suitable for biopharmaceutical production. CHO-gmt1 cells contain a dysfunctional CMP-sialic acid transporter,Citation10 CHO-gmt2 has a dysfunctional UDP-galactose transporter, and CHO-gmt3 has a dysfunctional GDP-fucose transporter. CHO-gmt5 cells have a double mutation leading to a dysfunctional CMP-sialic acid transporter and GDP-fucose transporter.Citation11 CHO-gmt4 cells are naturally occurring glycosylation mutants that were isolated from CHO-K1 cells using a cytotoxic lectin, Ricinus communis agglutinin-I (RCA-I). Genetic complementation tests revealed that all the RCA-I-resistant cells lack functional GnT I.Citation8 This is probably because RCA-I binds to many glycan structures, but has the least affinity for the Man5-GlcNAc2 structure.Citation12 In many CHO-gmt4 mutants we have analyzed so far, the GnT I loss-of-function mutation was due to a single point mutation in the GnT I coding region that resulted either in missense and nonsense mutations.Citation8 Theoretically, mammalian cells that are unable to transport UDP-GlcNAc into the Golgi or with a dysfunctional GnT I should both produce truncated N-glycans with the structure Man5-GlcNAc2. However, more than 100 RCA-I-resistant CHO mutants that we analyzed, all had lost GnT I activity (data not shown). None of the mutants was due to a mutated UDP-GlcNAc transporter. This may be due to the existence of several functionally redundant UDP-GlcNAc transporters in the mammalian cells.Citation13 No other glycosylation mutants were obtained using this lectin, demonstrating the specific use of RCA-I in isolating naturally occurring CHO mutants with dysfunctional GnT I.
The study of CHO glycosylation mutants was first pioneered by Stanley and coworkers.Citation14 Lec1 cells, which belong to this group of CHO mutants, do not possess GnT I activity. They were isolated using the lectin, Phaseolus vulgaris (L-PHA) from a Pro-5 CHO cell line and was used to determine the coding sequence of human GnT I.Citation15,Citation16 Other cell lines with dysfunctional GnT I have also been isolated. CHO-DUXK cells were also isolated using L-PHA lectin-resistant selection after undergoing ethyl methysulfonate (EMS) chemical mutagenesis. However, the authors found that these cells could not be adapted to protein-free suspension culture.Citation17 Another group utilized zinc-finger technology to inactivate the GnT I gene in CHO cells, leading to the generation of a panel of GnT I deficient cell lines that could be adapted to suspension culture and used for the production of recombinant proteins.Citation18 The authors also relied on the use of RCA-I to identify the mutant cells after the expression of the zinc-finger nuclease. GnT I deficient HEK293S cells have been isolated using EMS followed by lectin-resistant selection using ricin. The mutant cell line was used to produce a recombinant glycoprotein for crystal structure studies because the mutant produced highly homologous Man5GlcNAc2 N-glycans.Citation19 In another report, baby hamster kidney cells (BHK-21) that are deficient in GnT I due to single point mutations was isolated following treatment with chemical mutagenesis and lectin-resistant selection using ricin.Citation20
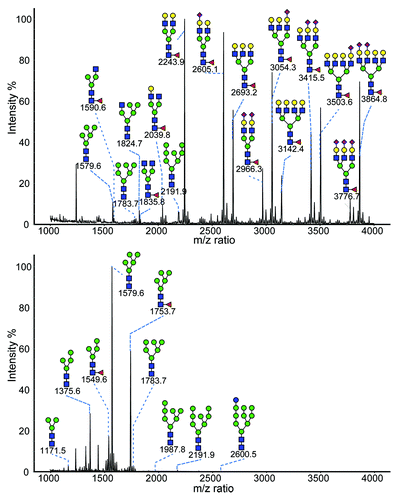
CHO-gmt4 cells were found to produce glycoproteins bearing glycans terminating in mannose residues. A fusion protein, EPO linked to IgG1 Fc (EPO-Fc), was transiently expressed in CHO-gmt4 and wild-type CHO-K1 cells. The N-glycans were cleaved from the respective EPO-Fc samples using PNGase F, purified, and permethylated before analysis using matrix-assisted laser desorption and/or ionization time-of-flight mass spectrometry (MALDI-TOF MS). The results are shown in . The N-glycans found on EPO-Fc produced in CHO-K1 are mostly of the complex type, with di-, tri- and tetra-antennary structures with incomplete sialylation. EPO-Fc produced by CHO-gmt4 cells contains afucosylated and fucosylated glycans with five mannose as the two major glycan species (Man5 and Man5F), followed by afucosylated and fucosylated glycans with four terminal mannose residues (Man4 and Man4F). The afucosylated glycans were in greater proportion to fucosylated glycans for both Man4 and Man5 glycans. Other pauci- and oligo-mannose N-glycans (Man4, Man6, Man7, Man8, and Glc1Man9) were also detected although at much lower relative abundance.
Superior Sialylation Observed in Transient and Stable Expression of EPO in CHO-gmt4
Interestingly, the sialylation of transiently expressed EPO produced in CHO-gmt4 with functionally restored GnT I was more superior to that produced in wild-type CHO-K1 cells. The better sialylation was not due to the overexpression of GnT I as the overexpression of GnT I in wild-type CHO-K1 did not show an improvement in sialylation.Citation8,Citation21 All CHO-gmt4 lines analyzed so far produced better sialylated EPO when functional GnT I was restored.Citation8 In addition, the restoration of GnT I function also led to the improved sialylation of recombinant EPO produced in Lec1 cells.Citation8 Only one kind of mutation in the GnT I coding sequence was found in each CHO-gmt4 line and this also holds true for the Lec1 cells.Citation8,Citation15 This may suggest that only one GnT I allele exists in CHO cells. The molecular mechanism for the enhanced sialylation remains to be discovered.
The overexpression of GnT I not only improved the sialylation of transiently expressed EPO in CHO-gmt4 cells, the enhanced sialylation was also observed for stably transfected CHO-gmt4 cells.Citation8 The detailed analysis of the glycan structures using high pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) revealed that this was due to a higher proportion of tri-and tetra-antennary branched glycans.Citation8 GnT I deficient CHO cell lines engineered with zinc-finger nuclease technology were also shown to have improved the sialylation of IgG1 molecules.Citation22
To extend the applicability of this cell line to industrial use, the mutant cell line was further engineered using zinc-finger nuclease technology targeting the dihydrofolate reductase gene (DHFR), resulting in a CHO-gmt4 cell line that can undergo methotrexate amplification. A panel of stable cell lines co-expressing EPO and GnT I showed that the superior EPO sialylation was maintained after gene amplification with methotrexate. Furthermore, the sialylation profile of EPO was also maintained when one of the stable cell lines was cultured in an industrial perfusion bioreactor. Purified EPO from this bioreactor run was analyzed using MALDI-TOF MS analysis, which showed a greater proportion of tri- and tetra-antennary sialylated glycans.Citation9 This result is in agreement with the earlier HPAEC-PAD analysis of transiently expressed EPO-Fc in CHO-gmt4 cells.Citation8
CHO-gmt4 cells Can Be Used to Produce Recombinant Proteins Targeting Cells with Mannose Receptors for Better Efficacy
Besides improving the efficacy of recombinant glycoproteins through better sialylation, the efficacy of other biologics can also be improved through the production of glycoproteins with more specific glycosylation for targeted cells.
In the treatment of Gaucher disease, recombinant glucocerebrosidase is produced to target macrophages that have been engorged due to the inability to metabolize glycolipids. Following production in CHO cells, purified glucocerebrosidase has to be enzymatically modified by glycosidases resulting in glycans containing terminal mannose residues for better efficacy as mannose-specific receptors are expressed on the surface of macrophages.Citation23,Citation24 Glucocerebrosidase produced in a human cell line using gene activation is called velaglucerase alfa (VPRIV). Its glycosylation is tailored to target macrophage cells by the addition of kifunensin, an inhibitor of α-mannosidase-I, resulting in the recombinant protein containing oligomannoses.Citation25 Taliglucerase alfa, glucocerebrosidase produced in carrot cells with terminal mannose glycansCitation26 has also been recently approved.
CHO-gmt4 is a good candidate host cell line for the production of glucocerebrosidase. The glycosylation mutant cells express recombinant glycoproteins with mannose-terminated N-glycans (), therefore bypassing the requirement for enzymatic deglycosylation to produce the mannose-terminated N-glycans. The use of kifunensine to alter the glycosylation pattern may affect cell growth and in addition, some complex N-glycan structures may still persist due to incomplete inhibition of the mannosidase enzyme.Citation27
Terminally-mannosylated proteins have been shown to elicit better immunogenicity as these mannosylated proteins are much more efficiently targeted to the mannose receptors of antigen-presenting cells such as dendritic cells.Citation28,Citation29 Hence CHO-gmt4 cells may potentially be a good host for producing cancer vaccines due to their ability to produce proteins containing terminal five-mannose sugars.
Conclusion
CHO-gmt4 cells are able to produce recombinant glycoproteins with two different glycosylation characteristics that cater to different applications. It can be used to produce therapeutic glycosylation with superior sialylation when functional GnT I is restored. CHO-gmt4 cells can also be used to produce glycoproteins with mannose-terminated N-glycans, such as glucocerebrosidase for Gaucher disease and protein-based cancer vaccines targeting the mannose receptors of antigen-presenting cells. We propose that the CHO-gmt4 glycosylation mutants represent a significant step toward the improvement in the quality of glycoprotein therapeutics production.
Disclosure of Potential Conflict of Interest
A*STAR has filed a patent application to cover the findings.
References
- Hudgin RL, Pricer WE Jr., Ashwell G, Stockert RJ, Morell AG. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem 1974; 249:5536 - 43; PMID: 4370480
- Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem 1971; 246:1461 - 7; PMID: 5545089
- Fukuda MN, Sasaki H, Lopez L, Fukuda M. Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood 1989; 73:84 - 9; PMID: 2910371
- Sodetz JM, Pizzo SV, McKee PA. Relationship of sialic acid to function and in vivo survival of human factor VIII/von Willebrand factor protein. J Biol Chem 1977; 252:5538 - 46; PMID: 301877
- Batta SK, Rabovsky MA, Channing CP, Bahl OP. Effect of removal of carbohydrate residues upon the half life and in vivo biological activity of human chorionic gonadotropin. Adv Exp Med Biol 1979; 112:749 - 56; http://dx.doi.org/10.1007/978-1-4684-3474-3_84; PMID: 572624
- Grabenhorst E, Schlenke P, Pohl S, Nimtz M, Conradt HS. Genetic engineering of recombinant glycoproteins and the glycosylation pathway in mammalian host cells. Glycoconj J 1999; 16:81 - 97; http://dx.doi.org/10.1023/A:1026466408042; PMID: 10612409
- Hossler P, Khattak SF, Li ZJ. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 2009; 19:936 - 49; http://dx.doi.org/10.1093/glycob/cwp079; PMID: 19494347
- Goh JSY, Zhang P, Chan KF, Lee MM, Lim SF, Song Z. RCA-I-resistant CHO mutant cells have dysfunctional GnT I and expression of normal GnT I in these mutants enhances sialylation of recombinant erythropoietin. Metab Eng 2010; 12:360 - 8; http://dx.doi.org/10.1016/j.ymben.2010.03.002; PMID: 20346410
- Goh JS, Liu Y, Liu H, Chan KF, Wan C, Teo G, Zhou X, Xie F, Zhang P, Zhang Y, et al. Highly sialylated recombinant human erythropoietin production in large-scale perfusion bioreactor utilizing CHO-gmt4 (JW152) with restored GnT I function. Biotechnol J 2014; 9:100 - 9; http://dx.doi.org/10.1002/biot.201300301; PMID: 24166780
- Lim SF, Lee MM, Zhang P, Song Z. The Golgi CMP-sialic acid transporter: A new CHO mutant provides functional insights. Glycobiology 2008; 18:851 - 60; http://dx.doi.org/10.1093/glycob/cwn080; PMID: 18713811
- Zhang P, Chan KF, Haryadi R, Bardor M, Song Z. CHO glycosylation mutants as potential host cells to produce therapeutic proteins with enhanced efficacy. Adv Biochem Eng Biotechnol 2013; 131:63 - 87; http://dx.doi.org/10.1007/10_2012_163; PMID: 23142953
- Iskratsch T, Braun A, Paschinger K, Wilson IB. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Anal Biochem 2009; 386:133 - 46; http://dx.doi.org/10.1016/j.ab.2008.12.005; PMID: 19123999
- Song Z. Roles of the nucleotide sugar transporters (SLC35 family) in health and disease. Mol Aspects Med 2013; 34:590 - 600; http://dx.doi.org/10.1016/j.mam.2012.12.004; PMID: 23506892
- Patnaik SK, Stanley P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol 2006; 416:159 - 82; http://dx.doi.org/10.1016/S0076-6879(06)16011-5; PMID: 17113866
- Kumar R, Yang J, Larsen RD, Stanley P. Cloning and expression of N-acetylglucosaminyltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc Natl Acad Sci U S A 1990; 87:9948 - 52; http://dx.doi.org/10.1073/pnas.87.24.9948; PMID: 1702225
- Chen W, Stanley P. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology 2003; 13:43 - 50; http://dx.doi.org/10.1093/glycob/cwg003; PMID: 12634323
- Zhong X, Cooley C, Seth N, Juo ZS, Presman E, Resendes N, Kumar R, Allen M, Mosyak L, Stahl M, et al. Engineering novel Lec1 glycosylation mutants in CHO-DUKX cells: molecular insights and effector modulation of N-acetylglucosaminyltransferase I. Biotechnol Bioeng 2012; 109:1723 - 34; http://dx.doi.org/10.1002/bit.24448; PMID: 22252477
- Sealover NR, Davis AM, Brooks JK, George HJ, Kayser KJ, Lin N. Engineering Chinese hamster ovary (CHO) cells for producing recombinant proteins with simple glycoforms by zinc-finger nuclease (ZFN)-mediated gene knockout of mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase (Mgat1). J Biotechnol 2013; 167:24 - 32; http://dx.doi.org/10.1016/j.jbiotec.2013.06.006; PMID: 23777858
- Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A 2002; 99:13419 - 24; http://dx.doi.org/10.1073/pnas.212519299; PMID: 12370423
- Opat AS, Puthalakath H, Burke J, Gleeson PA. Genetic defect in N-acetylglucosaminyltransferase I gene of a ricin-resistant baby hamster kidney mutant. Biochem J 1998; 336:593 - 8; PMID: 9841870
- Zhang P, Tan DL, Heng D, Wang T, Mariati, Yang Y, Song Z. A functional analysis of N-glycosylation-related genes on sialylation of recombinant erythropoietin in six commonly used mammalian cell lines. Metab Eng 2010; 12:526 - 36; http://dx.doi.org/10.1016/j.ymben.2010.08.004; PMID: 20826224
- Lin N, Davis D, Sealover NR, Mascarenhas J, George HJ, Kayser KJ. Mgat4 May Play a Role in Increased Sialylation by Overexpressing Functional MGAT1 in Mgat1-Disrupted Chinese Hamster Ovary (CHO) Cells. Poster presented at: BioProcess International™ Conference and Exhibition 2013; September 2013; Boston, MA, USA.
- Furbish FS, Steer CJ, Krett NL, Barranger JA. Uptake and distribution of placental glucocerebrosidase in rat hepatic cells and effects of sequential deglycosylation. Biochim Biophys Acta 1981; 673:425 - 34; http://dx.doi.org/10.1016/0304-4165(81)90474-8; PMID: 6784774
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE, et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med 1991; 324:1464 - 70; http://dx.doi.org/10.1056/NEJM199105233242104; PMID: 2023606
- Brumshtein B, Salinas P, Peterson B, Chan V, Silman I, Sussman JL, Savickas PJ, Robinson GS, Futerman AH. Characterization of gene-activated human acid-beta-glucosidase: crystal structure, glycan composition, and internalization into macrophages. Glycobiology 2010; 20:24 - 32; http://dx.doi.org/10.1093/glycob/cwp138; PMID: 19741058
- Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J 2007; 5:579 - 90; http://dx.doi.org/10.1111/j.1467-7652.2007.00263.x; PMID: 17524049
- Van Patten SM, Hughes H, Huff MR, Piepenhagen PA, Waire J, Qiu H, Ganesa C, Reczek D, Ward PV, Kutzko JP, et al. Effect of mannose chain length on targeting of glucocerebrosidase for enzyme replacement therapy of Gaucher disease. Glycobiology 2007; 17:467 - 78; http://dx.doi.org/10.1093/glycob/cwm008; PMID: 17251309
- Lam JS, Mansour MK, Specht CA, Levitz SM. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J Immunol 2005; 175:7496 - 503; http://dx.doi.org/10.4049/jimmunol.175.11.7496; PMID: 16301657
- Betting DJ, Mu XY, Kafi K, McDonnel D, Rosas F, Gold DP, Timmerman JM. Enhanced immune stimulation by a therapeutic lymphoma tumor antigen vaccine produced in insect cells involves mannose receptor targeting to antigen presenting cells. Vaccine 2009; 27:250 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.10.055; PMID: 19000731