?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Recombinant xylanase 2 from Penicillium occitanis expressed with an His-tag in Pichia pastoris, termed PoXyn2, was immobilized on nickel-chelate Eupergit C by covalent coupling reaction with a high immobilization yield up to 93.49%. Characterization of the immobilized PoXyn2 was further evaluated. The optimum pH was not affected by immobilization, but the immobilized PoXyn2 exhibited more acidic and large optimum pH range (pH 2.0–4.0) than that of the free PoXyn2 (pH 3.0). The free PoXyn2 had an optimum temperature of 50 °C, whereas that of the immobilized enzyme was shifted to 65 °C. Immobilization increased both pH stability and thermostability when compared with the free enzyme. Thermodynamically, increase in enthalpy and free energy change after covalent immobilization could be credited to the enhanced stability. Immobilized xylanase could be reused for 10 consecutive cycles retaining 60% of its initial activity. It was found to be effective in releasing reducing sugar from poultry feed. Immobilization on Eupergit C is important due to its mechanical resistance at high pH and temperature. Hence, considerable stability and reusability of bound enzyme may be advantageous for its industrial application.
Introduction
Xylan is the most abundant component of hemicelluloses in angiosperm plant cell walls and is the second most abundant carbohydrate in nature. Xylanases (EC 3.2.1.8; endo- β-1,4-D-xylanase) are mainly responsible for the hydrolysis of xylan with β-1,4-xylanolytic linkages.Citation1 Xylanases have been the focus of research owing to their industrial potential in many fields. They are, for instance, useful in pulp and paper industries, particularly for the facilitative role they play in the bleaching of craft pulp in order to reduce the amount of chlorine required for target pulp brightness.Citation2 A recent and exciting application of endoxylanases is the production of xylo-oligosaccharides with huge commercial value, because xylo-oligosaccharides, especially xylobiose, have been found to have a stimulatory effect on the selective growth of human intestinal Bifidobacteria, and are frequently defined as prebiotics.Citation3,Citation4 Another economically important application of Xyl-11 deals with animal feeding. For instance, in poultry production, xylanase supplementation in diet affects growth performance, digestion, and impact on immune parameters and gut microflora.Citation5 The utilization of thermostable enzymes might improve the technical and economic feasibility of all these biotechnological processes.
Because of the industrial potential of xylanases, a large number of studies have become interested in their immobilization for industrial application. Several advantages are gained through this technique and include the possibility of enzyme reuse, the enhancement of thermal stability, simplifying the product purification process, providing opportunities for scaling-up, and allowing the development of processes based on different reactor configurations and the reduction of operating costs.Citation6,Citation7
Covalent immobilization is often favored for biocatalyst production because the bonds formed are more stable than formed by electrostatic or adsorption.Citation8,Citation9 The epoxy supports can be used for covalent immobilization of enzymes and present several advantages; for instance, they are very stable during storage and they can increase the enzyme stability by preventing the enzyme from interactions with external interfaces (air, oxygen, immiscible organic solvents, etc.).Citation10,Citation11 Eupergit C which consists of macroporous beads with a diameter of 100–250 µm made by copolymerization of N,N-methylen-bis-(methacrylamide), glycidyl methacrylate, allyl glycidyl ether, and methacrylamide is very desirable for industrial scale enzyme immobilization because it is commercially available worldwide, resistant to mechanical and chemical stresses, and adaptable to a variety of configurations and specific processes performed in reactors.Citation12 Also, it may be very suitable to achieve the multipoint covalent attachment of enzymes, therefore, to stabilize their three-dimensional structure. Hence, it has been identified as the suitable carrier for covalent immobilization of enzymes for industrial applications.Citation8,Citation12,Citation13
Many reports on the immobilization of mesophilic and moderately thermophilic xylanases have been published.Citation12-Citation18
The Penicillium occitanis Pol6 xylanase termed PoXyn2 was successfully expressed with an His-tag in the methylotrophic yeast Pichia pastoris X-33 under the control of the glyceraldehyde 3-phosphate dehydrogenase (GAP) constitutive promoter.Citation19
Based on our recent workCitation20 the goal of this addendum is to study the effect of temperature and thermodynamics on the activity of free and immobilized enzyme and to further exploit it in increasing digestibility of poultry feed.
Effect of Temperature on Immobilized Xylanase
To study the temperature dependence, activities of the free and immobilized xylanases were determined at different temperatures at pH 3.0.
The effect of temperature on the activity of soluble and immobilized xylanase was determined by carrying out the reaction at various temperatures ranging from 30 to 75 °C. The temperature showing maximum activity was taken as optimum for the enzyme. The residual activity (%) at each temperature was calculated by considering the enzyme activity at the optimum temperature as 100%. The activation energy (Ea) of catalysis for both the free and immobilized xylanase forms was determined from the slope of the Arrhenius plot (log V [logarithm of % residual activity] vs. reciprocal of absolute temperature in Kelvin [1000/T]), which is given by the following expression:
To study the thermal stability, both free and immobilized enzyme forms were pre-incubated at different temperatures ranging from 50 to 75 °C up to 2 h. Samples were withdrawn at 30 min intervals and analyzed for activity in standard enzyme assays at the optimum temperature. The residual activity was calculated by taking the enzyme activity at 0 min incubation as 100%. Results were also expressed as first order thermal deactivation rate constants (kd), half-lives (t1/2) and D-values (decimal reduction time or time required to pre-incubate the enzyme at a given temperature to maintain 10% residual activity) at each temperature. The kd was determined by regression plot of log relative activity (%) vs. time (min). The t1/2 and D-value of immobilized xylanase were determined from the relationships.
The temperature rise necessary to reduce D-value by one logarithmic cycle (z-value) was calculated from the slope of graph between log D vs. T (°C) using the equation:
The activation energy (Ed) for xylanase denaturation was determined by a plot of log denaturation rate constants (ln kd) vs. reciprocal of the absolute temperature (K) using the equation:
The change in enthalpy (ΔH°, kJ mol−1), free energy (ΔG°, kJ mol−1) and entropy (ΔS°, J mol−1 K−1) for thermal denaturation of xylanase were determined using the following equation:
where T is the corresponding absolute temperature (K), R is the gas constant (8.314 J mol−1 K−1), h is the Planck constant (11.04 × 10−36 J min), and kB is the Boltzman constant (1.38 × 10−23 J K−1).
Results showed that optimum temperature values of 50 and 60 °C were recorded for free and immobilized enzyme, respectively (). A similar displacement of optimum temperature for immobilized enzymes has been observed in earlier studies but the extent of displacement varied from matrix to matrix and with the kind of interaction between enzyme and matrix.Citation21 The formation of intermolecular covalent bonds between the enzyme molecules and matrix support confer rigidity on the structure of the enzyme molecule, so that the enzyme is less affected by the denaturing effect of temperature.Citation22 The apparent activation energy (Ea) of catalysis for free and immobilized enzyme was calculated using Arrhenius plot (). The regression equations for Arrhenius plots of free and immobilized xylanases were y = −2.145x + 8.787 and y = −1.058x + 4.984, respectively. The covalent immobilization of PoXyn2 lowered the Ea from 17.8 to 8.8 kJ mol−1, resulting in a higher catalytic efficiency of xylanase.
Figure 1. Effect of temperature on free (solid circle) and immobilized (hollow circle) xylanase: (A) temperature optima, (B) Arrhenius plot to calculate activation energy (Ea) of catalysis, (C) stability of immobilized xylanase, (D) first order thermal deactivation of the immobilized xylanase, (E) Arrhenius plot to calculate activation energy (Ed) for denaturation, and (F) temperature dependence of the decimal reduction of free and immobilized xylanase to calculate z-values.
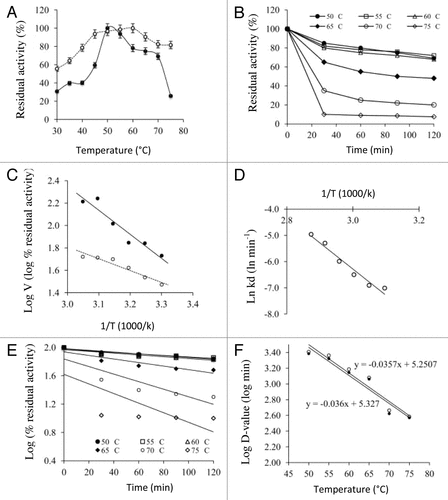
The thermal stability of the free and the immobilized PoXyn2 was studied in this work. It was seen that the free xylanase almost lost all its activity at 75 °C after 2 h of incubation (data not shown). However, xylanase immobilized on EupergitC preserved about 8% of its activity at 75 °C ().
The increase in temperature optima and stability upon immobilization might be the result of enzyme rigidity.Citation23 Similar increase in thermo-stability of several enzymes on immobilization has been observed.Citation24 In general, the immobilization process of the enzyme was suggested to protect the enzyme against heat inactivation.Citation25 The restricted interaction between the immobilized enzyme molecules could also be responsible for retaining the enzyme activity at higher temperatures.Citation26 Enhanced stability of xylanase at higher temperature is likely to increase its suitability for industrial application.Citation27
The rate of heat inactivation of immobilized enzyme was investigated in the temperature range between 50 °C and 75 °C. The plots of log (% remaining activity) vs. time were linear indicating the first order kinetics of immobilized enzyme (). The thermostability parameters of free and immobilized xylanase are summarized in . The half-lives and D-values of PoXyn2 xylanase prolonged remarkably at all temperatures after covalent attachment indicating better thermostability of the immobilized xylanase. Tyagi and GuptaCitation28 also reported an increase in the half-life of Aspergillus xylanase at 60 °C on immobilization. To determine the thermodynamic parameters for thermal stability, the activation energy (Ed) for thermal denaturation was determined by applying the Arrhenius plot ().
Table 1. Kinetic and thermodynamic parameters for thermal inactivation of free and immobilized xylanase
The Ed for immobilized enzyme was found 83.02 kJ mol−1, which was higher in comparison to 79.27 kJ mol−1 of free enzyme indicating that immobilized enzyme was very stable, compact, and highly resistant to heat denaturation. The higher value of Ed meant more energy was required to denature the treated enzyme as postulated earlier.Citation29 A similar increase in thermostability of several enzymes on immobilization was observed by researchers.Citation30 At 50 °C, the ΔH° of free xylanase was 73.94 kJ mol−1 while that of immobilized enzyme was 76.29 kJ mol−1 which clearly indicated that more energy was required for thermal denaturation of the immobilized enzyme. The values of ΔH° decreased with increasing temperature, in both cases, revealing that lesser energy was required to denature enzyme at high temperatures but still more in immobilized xylanase (). The observed change in ΔH° also indicated that enzyme in both states exhibited a considerable conformational change at higher temperatures.Citation31
The Gibbs free energy (ΔG◦) of thermal unfolding apparently increased with increase in temperature but did not reveal large differences between free and immobilized enzyme forms. This indicated that the immobilization of PoXyn2 did not adversely affect its thermal unfolding at higher temperatures. The unfolding of enzyme structure was accompanied with an increase in disorder or entropy of deactivation, but xylanase had negative entropy (ΔS◦) revealing that native form of enzyme was in more ordered state (). The immobilized enzyme also showed a negative value of ΔS° but the magnitude was substantially lesser as compared with free form. The less negative value for entropy in immobilized enzyme is normal due to the bonding.
Application of Immobilized Xylanase in Increasing Digestibility of Poultry Feed
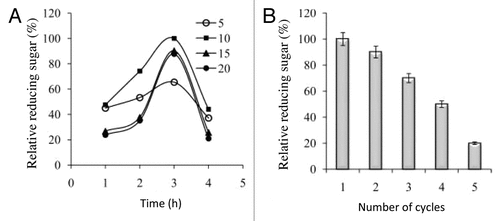
The poultry feed was treated with optimized conditions of immobilized enzyme dose and treatment time to measure the reducing sugar content in feed. The poultry feed was collected from local market and re-suspended in water with a ratio of 1:2. The resulting suspension of feed was treated with varying amounts of immobilized PoXyn2 (5–20 units/g feed) for different time intervals (1–6 h). The immobilized xylanase were recovered from the feed by centrifugation at 2000 rpm for 1 min to obtain a clear supernatant for estimation of reducing sugars using 3,5-dinitrosalycyclic acid. The recovered enzyme from the feed were washed with distilled water and added to the next batch of the feed for treatment. showed that the maximum reducing sugars were obtained with 10 units of immobilized PoXyn2 after an incubation of 3 h. On increasing the dose of xylanase from 10 to 20 units there was no significant effect on reducing sugar contents of the feed. Similarly, on increasing the incubation time after 3 h there was a decline in reducing sugars. The exact reason for decline in reducing sugars is not clear but, it may be due to chemical changes in reducing sugars. The enzyme inactivation during prolonged treatment may be another factor responsible for decline in sugar content. Further, the feed was treated with optimized dose of bound enzyme and resulted in 82 mg reducing sugar per gram feed. The immobilized xylanase could be reused for 5 consecutive cycles for the treatment of feed (). The recovery (%) of the immobilized xylanase after treating the poultry feed was 80.0 ± 5.0% at each cycle as compared with the preceding one. There is paucity of reports related to the enhancement in digestibility of poultry feed with immobilized xylanase. In fact, Maheshwari and ChandraCitation32 treated the starter feed with 5 units of xylanase, and resulted in release of 21 mg/ml of reducing sugar.
Conclusion
Herein, the covalent immobilization of His tagged xylanase from P.occitanis lowered the activation energy, resulting in a higher catalytic efficiency of xylanase. The half-life time of the immobilized enzyme was longer compared with the free enzyme. In general, the evolution of the half-life time is regarded as an indicator for the efficiency of the immobilization process. Accordingly, the immobilized xylanase is suitable in the feed industries as is effective in releasing reducing sugars. This certainly prompts further investigations on the continuous, particularly the large-scale, production of reducing sugars.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Wong KKY, Tan LUL, Saddler JN. Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 1988; 52:305 - 17; PMID: 3141761
- Gerber PJ, Heitmann JA, Joyce TW, Buchert J, Sii Kaaho M. Adsorption of hemicellulases onto bleached kraft fibers. J Biotechnol 1999; 67:67 - 75; http://dx.doi.org/10.1016/S0168-1656(98)00163-1
- Vázquez MJ, Alonso JL, Domínguez H, Parajó JC. Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol 2000; 11:387 - 93; http://dx.doi.org/10.1016/S0924-2244(01)00031-0
- Yanai T, Sato M. Purification and characterization of an beta-D-xylosidase from Candida utilis IFO 0639. Biosci Biotechnol Biochem 2001; 65:527 - 33; http://dx.doi.org/10.1271/bbb.65.527; PMID: 11330664
- Gao F, Jiang Y, Zhou GH, Han ZK. The effects of xylanase supplementation on growth, digestion, circulating hormone and metabolite levels, immunity and gut microflora in cockerels fed on wheat-based diets. Br Poult Sci 2007; 48:480 - 8; http://dx.doi.org/10.1080/00071660701477320; PMID: 17701501
- Sunna A, Antranikian G. Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol 1997; 17:39 - 67; http://dx.doi.org/10.3109/07388559709146606; PMID: 9118232
- Aksoy S, Tumturk H, Hasirci N. Stability of alpha-amylase immobilized on poly(methyl methacrylate-acrylic acid) microspheres. J Biotechnol 1998; 60:37 - 46; http://dx.doi.org/10.1016/S0168-1656(97)00179-X; PMID: 9571800
- Chaga GS. Twenty-five years of immobilized metal ion affinity chromatography: past, present and future. J Biochem Biophys Methods 2001; 49:313 - 34; http://dx.doi.org/10.1016/S0165-022X(01)00206-8; PMID: 11694287
- Ueda EK, Gout PW, Morganti L. Current and prospective applications of metal ion-protein binding. J Chromatogr A 2003; 988:1 - 23; http://dx.doi.org/10.1016/S0021-9673(02)02057-5; PMID: 12647817
- Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM. Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb Technol 2000; 26:509 - 15; http://dx.doi.org/10.1016/S0141-0229(99)00188-X; PMID: 10771054
- Martin MT, Plou FJ, Alcalde M, Ballesteros A. Immobilization on Eupergit C of cyclodextrin glucosyltransferase (CGTase) and properties of the immobilized biocatalyst. J Mol Catal, B Enzym 2003; 21:299 - 308; http://dx.doi.org/10.1016/S1381-1177(02)00264-3
- Katchalski-Katzir E, Kraemer DM. Eupergit® C, a carrier for immobilization of enzymes of industrial potential. J Mol Catal, B Enzym 2000; 10:157 - 76; http://dx.doi.org/10.1016/S1381-1177(00)00124-7
- Mateo C, Fernández-Lorente G, Cortés E, Garcia JL, Fernández-Lafuente R, Guisan JM. One-step purification, covalent immobilization, and additional stabilization of poly-His-tagged proteins using novel heterofunctional chelate-epoxy supports. Biotechnol Bioeng 2001; 76:269 - 76; http://dx.doi.org/10.1002/bit.10019; PMID: 11668463
- Pessela BCC, Mateo C, Carrascosa AV, Vian A, García JL, Rivas G, Alfonso C, Guisan JM, Fernández-Lafuente R. One-step purification, covalent immobilization, and additional stabilization of a thermophilic poly-His-tagged β-galactosidase from Thermus sp. strain T2 by using novel heterofunctional chelate-epoxy Sepabeads. Biomacromolecules 2003; 4:107 - 13; http://dx.doi.org/10.1021/bm020086j; PMID: 12523854
- Sardar M, Roy I, Gupta MN. Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer Eudragit(TM) L-100. Enzyme Microb Technol 2000; 27:672 - 9; http://dx.doi.org/10.1016/S0141-0229(00)00257-X; PMID: 11064049
- Ai ZL, Jiang ZQ, Li LT, Deng W, Kusakabe I, Li HS. Immobilization of Streptomyces olivaceoviridis E-86 xylanase on Eudragit S-100 for xylo-oligosaccharide production. Process Biochem 2005; 40:2707 - 14; http://dx.doi.org/10.1016/j.procbio.2004.12.006
- Gouda MK, Abdel-Naby MA. Catalytic properties of the immobilized Aspergillus tamarii xylanase. Microbiol Res 2002; 157:275 - 81; http://dx.doi.org/10.1078/0944-5013-00165; PMID: 12501991
- Lite L, Yunping Z, Zhigang H, Zhengqiang J, Weiwei C. Immobilization of the recombinant xylanase B (XynB) from the hyperthermophilic Thermotoga maritima on metal-chelate Eupergit C 250L. Enzyme Microb Technol 2007; 41:278 - 85; http://dx.doi.org/10.1016/j.enzmictec.2007.02.003
- Driss D, Bhiri F, Ghorbel R, Chaabouni SE. Cloning and constitutive expression of His-tagged xylanase GH 11 from Penicillium occitanis Pol6 in Pichia pastoris X33: purification and characterization. Protein Expr Purif 2012; 83:8 - 14; http://dx.doi.org/10.1016/j.pep.2012.02.012; PMID: 22402470
- Driss D, Haddar A, Ghorbel R, Chaabouni SE. Production of Xylooligosaccharides by Immobilized His-tagged Recombinant Xylanase from Penicillium occitanis on Nickel-Chelate Eupergit C. Appl Biochem Biotechnol 2014; http://dx.doi.org/10.1007/s12010-014-0932-0; PMID: 24801404
- Gawande PV, Kamat MYJ. Preparation, characterization and application of Aspergillus sp. xylanase immobilized on Eudragit S-100. J Biotechnol 1998; 66:165 - 75; http://dx.doi.org/10.1016/S0168-1656(98)00146-1; PMID: 9866868
- Martin MT, Alcalde M, Plou FJ, Dijkhuizen L, Ballesteros A. Synthesis of Malto-Oligosaccharides Via the Acceptor Reaction Catalyzed by Cyclodextrin Glycosyltransferases. Biotrans 2000; 19:21 - 35; http://dx.doi.org/10.3109/10242420109103514
- Ortega N, Perez-Mateos M, Pilar MC, Busto MD. Neutrase immobilization on alginate-glutaraldehyde beads by covalent attachment. J Agric Food Chem 2009; 57:109 - 15; http://dx.doi.org/10.1021/jf8015738; PMID: 19061308
- Tyagi R, Gupta MN. Immobilization of Aspergillus niger xylanase on magnetic latex beads. Biotechnol Appl Biochem 1995; 21:217 - 22; http://dx.doi.org/10.1111/j.1470-8744.1995.tb00332.x; PMID: 7718159
- Sardar M, Roy I, Gupta MN. Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer Eudragit(TM) L-100. Enzyme Microb Technol 2000; 27:672 - 9; http://dx.doi.org/10.1016/S0141-0229(00)00257-X; PMID: 11064049
- Wang P, Dai S, Waezsada SD, Tsao AY, Davison BH. Enzyme stabilization by covalent binding in nanoporous sol-gel glass for nonaqueous biocatalysis. Biotechnol Bioeng 2001; 74:249 - 55; http://dx.doi.org/10.1002/bit.1114; PMID: 11400098
- Agnihotri S, Dutt D, Tyagi CH, Kumar A, Upadhyaya JS. Production and biochemical characterization of a novel cellulase-poor alkali-thermo-tolerant xylanase from Coprinellus disseminatus SW-1 NTCC 1165. World J Microbiol Biotechnol 2010; 26:1349 - 59; http://dx.doi.org/10.1007/s11274-010-0307-9
- Tyagi R, Gupta MN. Immobilization of Aspergillus niger xylanase on magnetic latex beads. Biotechnol Appl Biochem 1995; 21:217 - 22; http://dx.doi.org/10.1111/j.1470-8744.1995.tb00332.x; PMID: 7718159
- Tayefi-Nasrabadi H, Asadpour R. Effect of Heat Treatment on Buffalo (Bubalus bubalis) Lactoperoxidase Activity in Raw Milk. J Biol Sci 2008; 8:1310 - 5; http://dx.doi.org/10.3923/jbs.2008.1310.1315
- Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 2007; 40:1451 - 63; http://dx.doi.org/10.1016/j.enzmictec.2007.01.018
- Marin E, Sanchez L, Perez MD, Puyol P, Calvo M. Effect of Heat Treatment on Bovine Lactoperoxidase Activity in Skim Milk: Kinetic and Thermodynamic Analysis. J Food Sci 2003; 68:89 - 93; http://dx.doi.org/10.1111/j.1365-2621.2003.tb14120.x
- Maheswari U, Chandra TS. Production and potential applications of a xylanase from a new strain of Streptomyces cuspidosporus. World J Microbiol Biotechnol 2000; 16:257 - 63; http://dx.doi.org/10.1023/A:1008945931108