Abstract
Gliomas are the most common primary intracranial tumors. Their distinct ability to infiltrate into the extracellular matrix (ECM) of the brain makes it impossible to treat these tumors using surgery and radiation therapy. A number of different studies have suggested that hyaluronan (HA), the principal glycosaminoglycan (GAG) in the ECM of the brain, is the critical factor for glioma invasion. HA-induced glioma invasion was driven by two important molecular events: matrix metalloproteinase (MMP) secretion and up-regulation of cell migration. MMP secretion was triggered by HA-induced focal adhesion kinase (FAK) activation, which transmits its signal through ERK activation and nuclear factor kappa B (NF-κB) translocation. Another important molecular event is osteopontin (OPN) expression. OPN expression by AKT activation triggers cell migration. These results suggest that HA-induced glioma invasion is tightly regulated by signaling mechanisms, and a detailed understanding of this molecular mechanism will provide important clues for glioma treatment.
Malignant gliomas are highly invasive and infiltrative tumors that have a poor prognosis with a median survival of only one year.Citation1,Citation2 A major barrier to effective malignant glioma treatment is the invasion of these cells into brain parenchyma. Because of this fact, local therapies such as surgery or radiation therapy are not effective.Citation3 Glioma cells invade through the ECM of the brain by activating a number of coordinated cellular programs, which include those necessary for migration and invasion.Citation3 Therefore, a detailed understanding of the mechanisms underlying this invasive behavior is essential for the development of novel effective therapies.
During glioma invasion, tumor cells closely interact with the ECM. Although brain tumor cells may share some of the invasive characteristics with tumors that arise outside of the central nerve system (CNS), the particular structure and composition of the brain ECM suggest the existence of unique invasive mechanisms for brain tumors.Citation4
Brain ECM is composed of typical ECM proteins and a HA scaffold with associated glycoproteins and proteoglycans.Citation5 Typical ECM proteins such as laminin, type-IV collagen and fibronectin have been implicated in the invasion of other tumors by regulating cell adhesion and migration.Citation6 However HA, which is associated with proteoglycans and GAGs, is especially abundant in the brain parenchyma compared to other tissues.Citation7 Furthermore, malignant gliomas contain higher amounts of HA than normal brain tissue.Citation7 These facts raise the possibility that HA might play an important role in glioma invasion, a process that is distinct from other non-CNS derived tumors.
HA Structure and Function
HA is a simple but unusual polysaccharide. It is a member of GAG family and is synthesized as a large, negatively charged, unbranched polymer that is composed of repeating disaccharides of glucuronic acid and N-acetylglucosamine.Citation8–Citation10 It has a simple chemical structure but differs in many ways from other GAGs. First, there are 10,000 or more disaccharide repeating units in HA, making it 103–104 kDa in molecular weight. Second, unlike other GAGs, which include heparan sulphate and chondroitin sulphate, HA contains no sulfate groups or epimerized uronic acid residues. Third, HA is synthesized at the inner face of the plasma membrane as a free linear polymer without any protein core, while other GAGs are synthesized by resident Golgi enzymes and covalently attached to core proteins.Citation10–Citation12
As an ECM component, HA plays a dual function. In normal ECM, HA provides tissue homeostasis, biomechanical integrity, and structure and assembly of tissues.Citation13 However in malignant tumor tissues, HA transmit signals into cytoplasm and increases tumor cell proliferation, motility and invasion.Citation14,Citation15 However, the molecular mechanism of HA-induced tumor progression is divergent and tissue specific.
Expression of HA in Cancer
HA contributes to certain types of cancer development. In addition to extracellular HA, intracellular and nuclear forms of HA have been detected. Intracellular HA is involved in cell signaling, whereas nuclear HA could promote chromatin condensation and thus facilitate mitosis.Citation16 HA expression is frequently increased in malignant tumors.Citation12 Histological studies demonstrate that HA concentrations are usually higher in malignant tumors than in corresponding benign or normal tissues.Citation12 HA levels can be increased around tumor cells themselves or within the tumor stroma.Citation7 Boregowda et al. reported that highly differentiated tumors such as lung, breast, colon, kidney, prostate, astrocytomas and non-Hodgkin's lymphoma expressed increased amounts of HA in both tumor epithelia and the intratumoral areas.Citation17
HA and Prognosis
The level of HA in tumor cells is predictive of malignancy and often correlates with cancer aggressiveness in patients with breast cancers, ovarian carcinomas, non-small-cell lung cancers and prostate cancer.Citation18–Citation22 For instance, a high proportion of HA-positive cancer cells and a high intensity of the HA-signal predicts a poor survival rate in colon carcinomas.Citation23 In addition, Auvinen et al. reported that both the intensity of stromal HA signal alone and that combined with the HA positivity in tumor cells were independent prognostic factors for overall survival in breast carcinoma patients.Citation18
HAS in Cancer
HA is made by HA synthases (HAS1, HAS2 and HAS3). Dysregulation of HAS genes results in abnormal production of HA and promotion of abnormal biological processes such as transformation and metastasis. It is thought that HA facilitates tumor growth by opening up spaces for the tumor to migrate to and by interacting with HA binding molecules, assisting in tumor cell adhesion and migration.Citation24 HA-induced tissue hydration physically creates spaces through which tumor cells may migrate and invade. HA-rich matrices within the tumor-associated stroma are also infiltrated by newly forming blood vessels.Citation12 A number of cancers are associated with elevated expression of HAS.Citation16,Citation25 In animal models, the overexpression of HAS promotes growth of fibrosarcoma and prostate carcinoma, as well as metastasis of mammary carcinoma.Citation26–Citation28
HA as a Cancer Marker
HA levels are often increased in the sera of patients with a variety of different tumors.Citation29–Citation31 It has also been shown that HA is significantly elevated in the sera of patients with metastatic disease compared to the sera of patients without metastatic disease.Citation29 In addition, Urinary HA and HAase levels correlate with levels that can be detected in tissues.Citation32 Likewise, elevated levels of HA and HAase in the urine form a clinically reliable marker for the presence and grade of bladder cancer and prostate cancer.Citation22,Citation32–Citation36 Therefore, HA is of considerable interest as a tumor marker in different cancers.
HA Receptors and Intracellular Signaling
Newly synthesized HA may be secreted and subsequently interact with several cell surface receptors such as cluster determinant 44 (CD44), receptor for hyaluronate-mediated motolity (RHAMM), lymphatic vessel endothelial HA receptor (LYVE-1), hyaluronan receptor for endocytosis (HARE), liver endothelial cell clearance receptor and Toll-like receptor-4. Of these, CD44 and RHAMM are well known as signal-transducing receptors that influence cell proliferation, survival and motility. Furthermore, they are known to be closely related to tumor progression.Citation37–Citation43
CD44
CD44, considered the principal receptor for HA, is a multifunctional single-pass transmembrane glycoprotein consisting of four functional domains: an amino terminal domain, stem structure, transmembrane domain and cytoplasmic domain.Citation44 The amino terminal domain contains motifs that provide docking sites for the ECM components such as HA and other GAGs.Citation45 The stem structure domain links the amino-terminal domain and transmembrane domain. Stem structure is enlarged by alternative splicing in cancer cells. Although some splice-forms include motifs for specific post-translational modification, little is known about the functional significance of an enlarged segment. The transmembrane region consists of 23 hydrophobic amino acids and cysteine residues which seem to be involved in oligomerization. This region might be responsible for the association of CD44 with lipid rafts. The cytoplasmic domain of CD44 has been linked to the cytoskeleton through interactions with proteins such as ankyrin and ERM proteins.Citation46,Citation47 This interaction has been implicated in cell adhesion and motility. Moreover, the cytoplasmic tail of CD44 interacts with many regulatory and adaptor molecules of cell signaling such as Src kinases, Rho GTPases, VAV2, GAB1, etc.Citation48 The interaction of CD44 with the cytoskeleton and various signaling molecules plays a pivotal role in promoting metastatic-specific tumor phenotypes such as MMP-mediated matrix degradation, tumor cell growth, migration and invasion.Citation12,Citation39,Citation41,Citation49–Citation51
RHAMM
RHAMM is also well-known as a HA-binding protein. RHAMM is expressed on the cell surface and in the cytoplasm, as well as in the cytoskeleton and nucleus. Like CD44, RHAMM is subject to alternative splicing, particularly during tissue repair or in cancer cells.Citation40,Citation52–Citation54 Interactions of HA with RHAMM can trigger a number of cellular signaling pathways including those that involve protein kinase C, FAK, MAP kinases, NFκB, RAS, phosphatidylinositol kinase (PI3K), tyrosine kinases and cytoskeletal components.Citation12,Citation48,Citation55–Citation58 Although it is clear that CD44 and RHAMM can participate independently in proliferative and migratory phenomena, their relative contributions to any given event have not been fully resolved in most cases, and it is likely that they have redundant or overlapping functions in some situations. In general, the interactions of HA with CD44 and RHAMM are especially important for tumorigenesis and tumor progression by activating downstream signaling molecules, but how this receptor transmits signal to downstream targets is still unclear.
Coupling with Other Receptors
CD44 is tightly coupled with receptor tyrosine kinases, p185HER2 and non-receptor tyrosine kinases, c-Src family kinases.Citation59,Citation60 CD44 and p185HER2 are physically linked to each other via interchain disulfide bonds and HA can stimulate CD44-associated p185HER2 tyrosine kinase activity that leads to increased tumor cell growth.Citation59 The cytoplasmic domain of CD44 binds to c-Src kinase at a single site with high affinity.Citation60,Citation61 Importantly, HA interaction with CD44 stimulates c-Src kinase activity, increasing tyrosine phosphorylation of cytoskeletal proteins such as cortactin. The binding of HA to CD44 isoforms, which complex with p185HER2 and c-Src kinase, likely triggers direct coupling with two tyrosine kinase-linked signaling pathways during tumor progression. This pathway can explain partly how HA receptors activate downstream targets to promote tumor progression.
Lipid Rafts
Although a number of binding partners of CD44 have been reported, the mechanisms of signal transduction via CD44 remain poorly understood. One of the most important signaling events following stimulation via CD44 is tyrosine phosphorylation of intracellular protein substrates. The Src-family non-receptor PTKs such as Lck, Fyn, Lyn and Hck were shown to be coupled to CD44.Citation62–Citation65 Moreover, CD44 resists solubilization in nonionic detergents, and recent investigations have shown that CD44 molecules lacking the cytoplasmic tail are still detergent insoluble, possibly because of their association with Triton X-100 insoluble lipids.Citation66,Citation67 These results provide an important link between the CD44 signaling pathway and lipid rafts because this behavior is similar to that of most glycosylphosphatidyl inositol (GPI) anchored glycoproteins, which are mostly confined to lipid rafts.Citation68 Co-isolation of CD44 with micro-domains strongly suggests that CD44 generates cellular activation signals utilizing the signaling machinery of the lipid rafts.
HA-induced MMP Secretion in Glioma Invasion
Glioblastoma is a severe type of primary brain tumor and irreversibly infiltrate the normal CNS by the interaction with ECM. One of the major component in brain ECM is HA, and glioma cells express high levels of the HA receptors, CD44 and RHAMM.Citation7,Citation69,Citation70 Both of these HA receptors were known to play an important role for glioma migration and invasion.Citation53,Citation69–Citation72
Another important player of glioma invasion is of MMPs. Several studies have demonstrated the increased expression of MMPs in gliomas, and GBM and anaplastic astrocytomas. They express higher levels of MMP-2 and -9 than do low-grade astrocytomas.Citation73 However, the relationship between HA signaling and MMP secretion was not well understood.
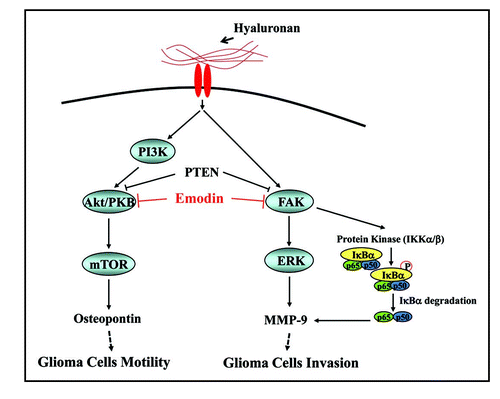
PTEN is one of the most frequently mutated tumor suppressors in GBM. PTEN also is known to suppresses glioma invasion by regulating MMP secretion.Citation74 Moreover, we reported that PTEN suppresses HA-induced secretion of MMP-9 and inhibits HA-induced invasion in U87MG cells, probably via dephosphorylation of FAK (see ).Citation74 We further addressed that FAK is direct substrate of PTEN as a protein phosphatase.Citation74 These results suggest that PTEN mutation is inevitable for the HA-induced glioma invasion by regulating MMP-9 secretion.
FAK, one of main substrates of the PTEN protein, is upregulated in GBMs, particularly in invasive zones. FAK overexpression has been correlated with the invasive potential of a tumor and poor patient prognosis.Citation75,Citation76 In HA-stimulated glioma invasion, FAK is required for the Ras-ERK 1/2 signaling pathway and modulates MMP-9 secretion ().Citation77,Citation78
Another important signaling event which regulates MMP expression is the transcriptional activation of genes whose promoter sequences contain putative binding sites for AP-1 and/or NFκB.Citation79 NFκB is in a family of transcription factors that have been shown to be involved in gene regulation of cellular processes like inflammation, immune response, cell proliferation and apoptosis.Citation80 NFκB activity is regulated by the endogenous inhibitor IκBα; interaction of NFκB with IκBα blocks the nuclear transport signal and keeps it sequestered in the cytoplasm. Following any kind of stimulation, IκBα is phosphorylated at serine residues 32 and 36, which leads to its ubiquitination and degradation. The free NFκB then translocates to nucleus and activates the transcription of target genes.Citation81 A number of upstream kinases such as NFκB-inducing kinase (NIK), PI3K and MEKK play significant roles in regulation of activation of IKK. In a recent study, we have demonstrated that HA elevates the levels of MMP-9 mRNA through NFκB activation ().Citation82 We further showed that HA-induced NFκB activation through phosphorylation and degradation of IκBα by activating IKK was mediated by FAK phosphorylation (). These results suggest that regulation of FAK is the most important step in blocking HA-induced MMP-9 secretion.
HA-Induced OPN Expression in Glioma Migration
As dicussed above, glioma invasion is enhanced not only by MMP secretion but also by upregulation of motility. Several studies have suggested that HA plays an important role in glioma cell motility and invasion, but the molecular mechanisms of HA-associated motility were not well understood.Citation74,Citation82 Recently, we identified group of genes which can be differentially regulated by HA treatment and demonstrated that OPN, one of the transcriptional targets of HA, is responsible for the stimulation of glioma motility by HA treatment ().Citation83 OPN, a highly phosphorylated glycoprotein of the ECM, is known to promote cell attachment, spreading and motility, as well as more complex events like vascular remodeling, bone mineralization and tumor metastasis by interacting with its cell surface receptors CD44 and the α-containing integrins.Citation84,Citation85 Interestingly, many of the basic cellular functions affected by OPN are also controlled by HA. Moreover, OPN and HA are frequently overexpressed in a variety of malignant cells and the expression levels of OPN and HA have been correlated with degrees of glioma malignancy.Citation86 These results suggest that OPN induction might be involved in the stimulation of HA-induced cell motility.
PTEN also suppresses migration, as genetic deletion of the PTEN tumor suppressor gene promotes cell motility and PTEN reconstitution or overexpression inhibits cell motility in a variety of cell type.Citation87,Citation88 The signaling mechanism that upregulates OPN expression was also under the control of PTEN activity.Citation85 OPN expression was upregulated by the PI3K/AKT/mTOR pathway in U87MG cells after treatment by HA in PTEN deleted glioma cells and reconstitution of PTEN suppressed OPN expression ().Citation83 These results suggest that PTEN suppress glioma motility and invasion by the inhibition of HA-induced OPN expression and MMP-9 secretion.
Emodin as an Anti-Cancer Drug Against Glioma Invasion
Since HA is a primary ECM component that can stimulate glioma invasion, it makes inhibitors of this signaling as attractive candidates for therapeutic agents. Among protein tyrosine kinase inhibitors, we found that emodin (3-methyl-1,6,8-trihydroxyanthraquinone) has strong anti-invasive activity for HA-induced glioma invasion ().Citation82 Emodin is one of the main active components contained in the root and rhizome of Rheum palmatum L. Emodin has been shown to have a number of biological activities, including antiviral, antimicrobial, immunosuppressive, hepatoprotective, anti-inflammatory and anticancer effects.Citation89–Citation91 Especially in glioma cells, emodin was known to suppress the TGFbeta and FGF-2 induced expression of syndecan-1, a major cell surface heparin sulfate proteolycan, which was specific to malignant gliomas and was absent in normal brain tissues and it may participate in the motility of glioma cells.Citation92 Emodin suppressed the expression of MMP-2 and MMP-9 at both transcriptional and translational levels.Citation82 Pharmacological studies indicated that emodin can significantly decrease the activation of FAK, AKT and ERK1/2, thereby suppressing MMP production ().Citation82 We further observed that emodin efficiently suppressed the HA-stimulated AP-1 and NFκB promoter activities.Citation82 In an in vivo tumor progression model, the activity of MMP-9 was suppressed by emodin treatment.Citation82 These results indicate that oral administration of emodin effectively suppresses MMP-9 expression in human glioma through inhibition of AKT and ERK1/2 activation in vivo. These findings suggest that emodin may be a clinically valuable anti-cancer agent against gliomas by blocking FAK and AKT activation, which is the key signaling event of HA-induced MMP secretion and cell motility.
Conclusions and Future Directions
Understanding of molecular mechanisms of glioma invasion is critical in developing novel therapeutic strategies or treatments because major therapeutic approaches such as surgery and radiotherapy are useless without blocking glioma invasion. In this review, we have demonstrated that HA is a major player in the brain ECM that cooperates with PTEN-deletion during glioma invasion. These results provide strong evidence for the emerging concept in cancer research that the microenvironment modulates the effects of genetic alterations.
PTEN is a tumor suppressor that plays important roles by blocking HA-induced FAK activation and AKT activation. FAK and AKT activation is important for the secretion of MMP and motility of cells. By using emodin, a protein tyrosine kinase inhibitor, we have demonstrated that blocking HA signaling partially reduced tumor size. These results suggest that chemotherapeutic treatments that can effectively block HA induced signaling might be clinically valuable as an anti-invasive agent for gliomas.
Figures and Tables
Figure 1 Inhibition mechanism of emodin on HA-induced glioma invasion and motility. HA induced the invasion of glioma cells by the induction of MMP-9 through the RaS/FAK/ERK 1, 2 activation. In addition, NFκB translocation by FAK activation is also important for the MMP-9 expression. PTEN can effectively modulate the expression of MMP-9 by the dephosphorylation of FAK. HA induced the motililty of glioma cells by the induction of OPN through the PI3K/AKT/mTOR pathway. PTEN can effectively modulate the expression of OPN, and induced OPN contributes to HA-induced cell migration in glioma cells. Emodin suppresses HA-induced MMP-9 and OPN expression through the inhibition of FAK, and AKT activation. Emodin as a protein tyrosine kinase inhibitor can block protein kinases which are important for the HA-induced glioma invasion and motility.
Acknowledgements
This work was supported by the National Cancer Center Grant 0410052-3.
References
- Scott JN, Rewcastle NB, Brasher PM, Fulton D, MacKinnon JA. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol 1999; 46:183 - 188
- Senger D, Cairncross JG, Forsyth PA. Long-term survivors of glioblastoma: Statistical aberration or important unrecognized molecular subtype?. Cancer J 2003; 9:214 - 221
- Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery 1996; 39:235 - 250
- Clerck YA, Shimada H, Gonzalez-Gomez I, Raffel C. Tumoral invasion in the central nervous system. J Neuro-Oncol 1994; 18:111 - 121
- Rutka JT, Apodaca G, Stern R, Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg 1988; 69:155 - 170
- Goldbrunner RH, Haugland HK, Klein CE, Kerkau S, Roosen K, Tonn JC. ECM dependent and integrin mediated tumor cell migration of human glioma and melanoma cell lines under serum free condition. Anticancer Res 1996; 16:3679 - 3688
- Delpech B, Maingonnat C, Girard N, Chauzy C, Maunoury R, Olivier A, Tayot J, Creissard P. HA and hyaluronectin in the extracellular matrix of human brain tumor stroma. Eur J Cancer 1993; 29:1012 - 1017
- Laurent TC. Balazs EA. The structure of hyaluronic acid. Chemistry and Molecular Biology of the Intercellular Matrix 1970; New York Academic Press 703 - 732
- Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992; 6:2397 - 2404
- Scott JE, Heatley F. Biological properties of hyaluronan in aqueous solution are controlled and sequestered by reversible tertiary structures, defined by NMR spectroscopy. Biomacromolecules 2002; 3:547 - 553
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megadalton, stealth molecule. Current Opinion in Cell Biology 2000; 12:581 - 586
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nature Reviews. Cancer 2004; 4:528 - 539
- Balazs EA, Denlinger JL. Clinical uses of hyaluronan. Ciba Found Symp 1989; 143:265 - 280
- Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science 1998; 280:1036 - 1037
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70
- Adamia S, Treon SP, Reiman T, Tournilhac O, McQuarrie C, Mant MJ, Belch AR, Pilarski LM. Potential impact of a single nucleotide polymorphism in the hyaluronan synthase 1 gene in Waldenstroma's macroglobulinemia. Clin Lymphoma 2005; 4:253 - 256
- Boregowda RK, Appaiah HN, Siddaiah M, Kumarswamy SB, Sunila S, Thimmaiah KN, Mortha K, Toole B, Banerjee S. Expression of hyauronan in human tumor progression. J Carcinog 2006; 5:2
- Auvinen P, Tammi R, Parkkinen J, Tammi M, Ågren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma VM. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 2000; 156:529 - 536
- Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 2000; 60:150 - 155
- Pirinen R, Tammi R, Tammi M, Hirvikoski P, Parkkinen JJ, Johansson R, Böhm J, Hollmén S, Kosma VM. Prognostic value of hyaluronan expression in non-small-cell lung cancer: increased stromal expression indicates unfavorable outcome in patients with adenocarcinoma. Int J Cancer 2001; 95:12 - 17
- Lipponen P, Aaltomaa S, Tammi R, Tammi M, Agren U, Kosma VM. High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. Eur J Cancer 2001; 7:849 - 856
- Posey JT, Soloway MS, Ekici S, Sofer M, Civantos F, Duncan RC, Lokeshwar VB. Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res 2003; 63:2638 - 2644
- Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Ågren U, Alhava E, Kosma VM. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998; 58:342 - 347
- Stuhlmeier KM. Aspects of the biology of hyaluronan, a largely neglected but extremely versatile molecule. Wien Med Wochenschr 2006; 156:563 - 568
- Liu N, Gao F, Han Z, Xu X, Underhill CB, Zhang L. Hyaluronan synthase 3 overexpression promotes the growth of TSU prostate cancer cells. Cancer Res 2001; 61:5207 - 5214
- Itano N, Sawai Y, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 1999; 274:25085 - 25092
- Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol 2002; 161:849 - 857
- Heldin P. Importance of hyaluronan biosynthesis and degradation in cell differentiation and tumor formation. Braz J Med Biol Res 2003; 36:967 - 973
- Kumar S, West DC, Ponting JM, Gattamaneni HR. Sera of children with renal tumours contain low-molecular-mass hyaluronic acid. Int J Cancer 1989; 44:445 - 448
- Thylen A, Wallin J, Martensson G. Hyaluronan in serum as an indicator of progressive disease in hyaluronan-producing malignant mesothelioma. Cancer 1999; 86:2000 - 2005
- Hasselbalch H, Hovgaard D, Nissen N, Junker P. Serum hyaluronan is increased in malignant lymphoma. Am J Hematol 1995; 50:231 - 233
- Hautmann SH, Lokeshwar VB, Schroeder GL, Civantos F, Duncan RC, Gnann R, Friedrich MG, Soloway MS. Elevated tissue expression of hyaluronic acid and hyaluronidase validates the HA-HAase urine test for bladder cancer. J Urol 2001; 165:2068 - 2074
- Lokeshwar VB, Schroeder GL, Selzer MG, Hautmann SH, Posey JT, Duncan RC, Watson R, Rose L, Markowitz S, Soloway MS. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests. Cancer 2002; 95:61 - 72
- Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, Friedrich MG, Ekici S, Huland H, Lokeshwar V. A side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol 2004; 172:1123 - 1126
- Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL. HYAL1 hyaluronidase in prostate cancer: a tumor promoter and suppressor. Cancer Res 2005; 65:7782 - 7789
- Lokeshwar VB, Cerwinka WH, Lokeshwar BL. HYAL1 hyaluronidase: a molecular determinant of bladder tumor growth and invasion. Cancer Res 2005; 65:2243 - 2250
- Laurent TC, Laurent UB, Fraser JR. Serum hyaluronan as a disease marker. Annal Med 1996; 28:241 - 253
- Vercruysse KP, Ziebell MR, Prestwich GD. Control of enzymatic degradation of hyaluronan by divalent cations. Carbohydrate Res 1999; 318:26 - 37
- Toole BP. Hyaluronan promotes the malignant phenotype. Glycobiology 2002; 12:37 - 42
- Turley EA, Noble PW, Bourguignon LYW. Signaling properties of hyaluronan receptors. J Bio Chem 2002; 277:4589 - 4592
- Spicer AP, Tien JYL. Hyaluronan and morphogenesis. Birth Defects Res C 2004; 72:89 - 108
- Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic target in cancer. Current Drug Targets. Cardiovasc Haematol Dis 2005; 5:3 - 14
- Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J 2006; 20:9 - 22
- Stamenkovic I, Amiot M, Pessando JM, Seed BA. Lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 1989; 56:1057 - 1062
- Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem 2002; 277:4585 - 4588
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 1994; 126:391 - 401
- Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44 (GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol 1994; 126:1099 - 1109
- Ponta H, Sherman L, Herrlich P. CD44: from adhesion molecules to signalling regulators. Nature Rev Mol Cell Biol 2003; 4:33 - 45
- Li H, Guo L, Li JW, Liu N, Qi R, Liu J. Expression of hyaluronan receptors CD44 and RHAMM in stomach cancers: relevance with tumor progression. Int J Oncol 2000; 17:927 - 932
- Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biology 2002; 21:15 - 23
- Knudson CB. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C 2003; 69:174 - 196
- Zhang S, Chang MC, Zylka D, Turley S, Harrison R, Turley EA. The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J Biol Chem 1998; 273:11342 - 11348
- Akiyama Y, Jung S, Salhia B, Lee S, Hubbard S, Taylor M, Mainprize T, Akaishi K, van Furth W, Rutka JT. Hyaluronate receptors mediating glioma cell migration and proliferation. J Neuro-Oncol 2001; 53:115 - 127
- Lynn BD, Turley EA, Nagy JI. Subcellular distribution, calmodulin interaction and mitochondrial association of the hyaluronan-binding protein RHAMM in rat brain. J Neurosci Res 2001; 65:6 - 16
- Hall CL, Lange LA, Prober DA, Zhang S, Turley EA. Pp60 (c-src) is required for cell locomotion regulated by the hyaluronan receptor RHAMM. Oncogene 1996; 13:2213 - 2224
- Fieber C, Plug R, Sleeman J, Dall P, Ponta H, Hofmann M. Characterisation of the murine gene encoding the intracellular hyaluronan receptor IHABP (RHAMM). Gene 1999; 226:41
- Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, Longati P, Velten FW, Johansson S, Goerdt S. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J 2002; 362:155 - 164
- Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in coordinating adhesive and signaling events. J Cell Sci 2004; 117:373 - 380
- Bourguignon LY, Gunja-Smith Z, Iida N, Zhu HB, Young LJ, Muller WJ, Cardiff RD. CD44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol 1998; 176:206 - 215
- Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem 2001; 276:7327 - 7336
- Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8–10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton 1999; 43:269 - 287
- Herrlich P, Zoller M, Pals ST, Ponta H. CD44 splice variants: Metastases meet lymphocytes Immunol Today 1993; 14:395 - 399
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990; 61:1303 - 1313
- Taher TE, Smit L, Griffioen AW, Schilder Tol EJ, Borst J, Pals ST. Signaling through CD44 is mediated by tyrosine kinases. Association with p56lck in T lymphocytes. J Biol Chem 1996; 271:2863 - 2867
- Lisanti MP, Scherer PE, Vidugiriene J, Tang ZL, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source. Implications for human disease. J Cell Biol 1994; 126:111 - 126
- Neame SJ, Uff CR, Sheikh H, Wheatley SC, Isacke CM. CD44 exhibits a cell type dependent interaction with triton X-100 insoluble, lipid rich, plasma membrane domains. J Cell Sci 1995; 108:3127
- Perschl A, Lesley J, English N, Hyman R, Trowbridge IS. Transmembrane domain of CD44 is required for its detergent insolubility in fibroblasts. J Cell Sci 1995; 108:1033 - 1041
- Low MG. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta 1989; 988:427 - 454
- Radotra B, McCormick D. Glioma invasion in vitro is mediated by CD44-hyaluronan interactions. J Pathol 1997; 181:434 - 438
- Merzak A, Koocheckpour S, Pilkington GJ. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res 1994; 54:3988 - 3992
- Gilg AG, Tye SL, Tolliver LB, Wheeler WG, Visconti RP, Duncan JD, Kostova FV, Bolds LN, Toole BP, Maria BL. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res 2008; 14:1804 - 1813
- Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci 2002; 9:282 - 288
- Uhm JH, Dooley NP, Villemure JG, Yong VW. Mechanisms of glioma invasion: role of matrix-metalloproteinases. Can J Neurol Sci 1997; 24:3 - 15
- Park MJ, Kim MS, Park IC, Kang HS, Yoo H, Park SH, Rhee CH, Hong SI, Lee SH. PTEN suppresses hyaluronic acid-induced matrix metalloproteinase-9 expression in U87MG glioma cells through focal adhesion kinase dephosphorylation. Cancer Res 2002; 21:6318 - 6322
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 2000; 6:2417 - 2423
- Recher C, Ysebaert L, Beyne-Rauzy O, Mansat-De Mas V, Ruidavets JB, Cariven P, Demur C, Payrastre B, Laurent G, Racaud-Sultan C. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity and poor prognosis. Cancer Res 2004; 64:3191 - 3197
- Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem 1997; 272:13189 - 13195
- Mon NN, Hasegawa H, Thant AA, Huang P, Tanimura Y, Senga T, Hamaguchi M. A role for focal adhesion kinase signaling in tumor necrosis factor-alpha-dependent matrix metalloproteinase-9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res 2006; 66:6778 - 6784
- Yokooo T, Kitammura M. Dual regulation of IL-1b-mediated matrix metalloproteinase-9 expression in mesangial cells by NFκB and AP-1. Am J Physiol 1996; 270:123 - 130
- Ghosh S, May M, Kopp E. NFkappaB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998; 16:225 - 260
- Baichwal VR, Baeuerle PA. Activate NFkappaB or die?. Curr Biol 1997; 7:94 - 96
- Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H, Shin SH, Song ES, Lee SH. Emodin suppresses hyaluronic acid-induced MMP-9 secretion and ivnasion of gliomas cells. Int J Oncol 2005; 27:839 - 846
- Kim MS, Park MJ, Moon EJ, Kim SJ, Lee CH, Yoo H, Shin SH, Song ES, Lee SH. Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/AKT pathway to enhance the motility of human glioma cells. Cancer Res 2005; 65:686 - 691
- Denhardt DT, Mistretta D, Chambers AF. Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter. Clin Exp Metastasis 2003; 20:77 - 84
- Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW. A biochemical characterization of the binding of osteopontin to integrins avb1 and avb5. J Biol Chem 1995; 270:26232 - 26238
- Saitoh Y, Kuratsu J, TAkeshima H, Yamamoto S, Ushio Y. Expression of osteopontin in human glioma. Its correlation with the malignancy. Lab Invest 1995; 72:55 - 63
- Liliental J, Moon SY, Lesche R. Genetic deletionof the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol 2000; 6:401 - 404
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading and focal adhesion by tumor suppressor PTEN. Science 1998; 280:1614 - 1617
- Koyama M, Kelly TR, Watanabe KA. Novel type of potential anticancer agents derived from chrysophanol and emodin. J Med Chem 1988; 31:283 - 284
- Huang HC, Chu SH, Chao L. Vasorelaaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur J pharmacol 1991; 198:211 - 213
- Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol 2004; 68:361 - 371
- Arata Watanabe A, Mabuchi T, Satoh E, Furuya K, Zhang L, Maeda S, Maeda S, Naganuma H. Expression of syndecans, a heparin sulfate proteoglycan, in malignant gliomas: participation of nuclear factor-kappaB in upregulation of syndecan-1 expression. J Neurooncol 2006; 77:25 - 32