Abstract
Members of the integrin family of cell adhesion receptors are pivotal to the formation of complex tissues and organs in animals. They mediate cell adhesion by interacting with the extracellular matrix and by binding to intracellular linker proteins that connect to the cytoskeleton. We have recently identified a new and evolutionarily conserved component of the linker complex, the Drosophila Wech protein. Wech is essential for embryonic muscle attachment. It belongs to the RBCC/TRIM family of cytoplasmic multidomain proteins and contains a carboxyterminal NHL domain. Wech protein is specifically localized to the embryonic muscle attachment sites and wech mutant embryos show muscle detachment from the body wall. In β-integrin or talin mutants Wech is mislocalized, as the localization of Integrin-linked-kinase (ILK) depends on Wech. Biochemical data indicate that Wech is associated with the head domain of Talin and the kinase domain of ILK suggesting that Wech may be involved in the linkage of both core proteins of the linker complex. We discuss that Wech proteins may be crucial and evolutionarily conserved regulators of cell-type specific integrin functions and that their activities may underlie complex regulation by microRNAs.
Tissue and organ formation in animals involves adhesion between different cell layers and the extracellular matrix which is often mediated by members of the integrin family of heterodimeric transmembrane receptors.Citation1 In Drosophila, the functional role of integrins in adhesion and signalling has been extensively studied for the attachment of somatic and visceral muscles, morphogenesis of the gut and epidermis, and adhesion between the two surfaces of the wing blade.Citation2 During the formation of the epidermal muscle attachment sites, the muscles attach to a specialized epidermal cell called the tendon cell.Citation3 Dense hemiadherens-type junctions are formed between the tendon cell, the muscle and the extracellular matrix that is deposited in between them. These tight junction connections are essential to transmit the force of the muscles to the exoskeleton allowing for animal movement. Loss of cell adhesion by aberrant integrin expression leads to muscle detachment in flies and mice.Citation2–Citation4
A crucial part of the adhesive function of integrins is their ability to connect to the actin cytoskeleton. This involves a complex of adaptor proteins, which bind to the cytoplasmic tail of integrins and mediate the link to the cytoskeleton.Citation5 Proteins of this link are organized into a multiprotein-complex which has been named “the adhesome” that is estimated to contain more than 150 proteins.Citation6 One of the essential and evolutionarily conserved components of the link is Talin. Absence of Talin causes almost identical defects to the absence of integrins, such as muscle detachment and failure of germband retraction during embryonic development.Citation7 This is in contrast to other components of the link, such as Integrin-linked-kinaseCitation8 (ILK), PINCHCitation9 or Tensin,Citation10 whose absence cause only a subset of the defects. In different types of cells, integrins make diverse connections with the actin cytoskeleton, however, the molecular basis for the cell-type-specific functions of integrins is not well known. We have recently identified a new component of the link which may represent an evolutionarily conserved cell-type specific regulator of integrin functions in Drosophila, the wech geneCitation11 (the wech locus is also named dappled).
The wech gene encodes a multidomain protein of the super-family of RBCC (Ring-B-box-Coiled-Coil)/tripartite motif (TRIM) proteins.Citation12 It contains a B box zinc-finger, a coiled-coil domain and a protein motif consisting of 5 or 6 NHL (NCL-1, HT2A, LIN-41) repeats (in short called the NHL domain).Citation13 All of these domains mediate protein-protein interactions and are contained in various proteins across species, however, the combination of domains is found in only four proteins in Drosophila. These include Wech,Citation11 the tumor suppressor brain tumor (Brat),Citation14 the meiotic protein Mei-P26,Citation15 and the newly identified Another B-Box Affiliate (ABBA) protein whose function is still unknown.Citation16 Single copy genes of wech orthologs are found in other invertebrates and in mammals, including mice, rats and humans. The C. elegans Wech ortholog is named Lin-41 and is involved in the regulation of the progression from L4 to the adult developmental program.Citation17
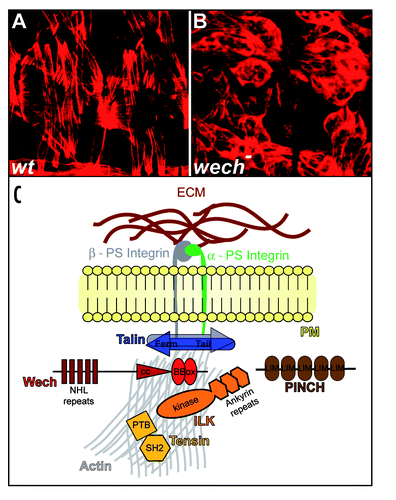
Phenotypic analysis of Drosophila embryos carrying a homozygous wech allele in which both the wech transcript and protein levels are strongly reduced, reveals that muscles are detached from the body wall in late embryonic stagesCitation11 (wech is a German Rhineland dialect for “detached” or “gone”). This muscle detachment phenotype is remarkably similar to mutants for β-integrin or talin ( and B).Citation7,Citation18,Citation19 During early stages of embryonic development Wech protein is expressed ubiquitously in all epithelial cells, and after germband retraction it increasingly accumulates very specifically in muscle attachment sites where it is found in a cortical localization in both the epidermal tendon cells and the muscle cells.Citation11 βPS Integrin and components of the cytoplasmic integrin-linked complex, Talin, ILK and Tensin, which bind to the cytoplasmic tail of βPS Integrin, co-localize with Wech. Both βPS Integrin and Talin are required for Wech localization. In contrast, Wech is required for ILK and Tensin localization at the attachment sites. PINCH, which was shown to modulate ILK function by direct binding or by recruitment of an ILK-modifying factor,Citation9 is still localized properly in wech mutants. Altogether, the data suggest that Wech may be required to link an ILK-containing multiprotein complex to Talin, thereby providing a link between integrins and the cytoskeleton in the muscle attachment sites. PINCH apparently acts in parallel to Wech and may contribute to the assembly of an ILK-containing complex (). Biochemical analysis further supports this hypothesis since it was found by immunoprecipitation that Wech is associated with the head domain of Talin and the kinase domain of ILK. It is not yet known whether the interactions of Wech with ILK and Talin are direct or whether additional proteins are involved. The finding that the wech mutant phenotype is stronger than the one of ILK mutants suggests that other unknown factors, in addition to ILK, may depend on Wech function during muscle attachment.
Is Wech a general regulator of integrin-dependent processes? Many components of the integrin-cytoskeleton linker complex, including Talin, PINCH and Tensin have been identified and characterized in the Drosophila wing.Citation20 In the wing, loss of integrin functions cause the separation of the two layers of the wing resulting in a wing blister phenotype.Citation21 Although wech mRNA expression has been observed in the wing disc,Citation16 our unpublished observations indicate that Wech protein is not significantly expressed in the wing. Furthermore, we have not observed wing blister phenotypes in wech RNAi knock down animals. It is therefore possible that Wech may not be functionally involved in integrin-dependent adhesion of the wing layers. Furthermore, other phenotypes of β-integrin or talin mutants, such as impaired germband retraction or a failure of adhesion of the visceral mesoderm to the gut endoderm, are not evident in wech mutants. Thus it is likely that Wech may be involved in cell-specific integrin functions rather than being a general regulator of integrin-dependent processes. Tissue-specific integrin functions have also been demonstrated for other components of the integrin pathway, including Tensin which was demonstrated to be essential for integrin-dependent adhesion of the wing layers, but which is not pivotal for integrin-dependent muscle attachment.Citation10 These data are in support of the “toolkit” hypothesis put forward by Delon and BrownCitation22 which suggests that the components of the link are differentially used in various tissues and developmental situations, like a “toolkit.” In the muscle attachment sites, Wech may be required to specifically strengthen the adhesive junctions between muscles and tendon cells or assemble yet unknown factors that are functionally dependent on integrin-mediated adhesion in their control function during muscle attachment. A similar scenario may also apply to the single Wech orthologs in vertebrates for which, however, no functional studies are available yet. The murine, the human and the chicken Wech ortholog (named TRIM71/Lin41) also possess a B-box, a coiled-coil domain, and a carboxyterminal NHL-domain which shows a sequence similarity of more than 60% as compared to the Drosophila Wech protein.Citation11,Citation23 In mouse and chicken, the mRNA of the wech orthologs is expressed in the developing limb buds, brachial arches, the eyespot, the developing muscles and the developing brain.Citation23–Citation25 These tissues represent a subset of tissues for which integrin-functions have been described which may also point towards a more tissue and cell-specific function of vertebrate Wech proteins in integrin-dependent processes.
Apart from the putatively conserved function of Wech proteins in the integrin-cytoskeleton link, there are a number of particularities about the Drosophila wech gene locus and its regulation that may point to additional roles of Wech proteins in organ development and physiology. We know from Drosophila wech that its mRNA and protein expression patterns are not identical. wech mRNA is strongly expressed, for example, in the developing peripheral and central nervous system, however, we have not observed significant Wech protein expression in these tissues in the embryo.Citation11 A similar phenomenon is known for Drosophila Talin, for which a discrepancy of embryonic mRNA and protein expression was also described. talin mRNA is also strongly expressed in the PNS and CNS, however protein is not significantly expressed in these tissues.Citation7 Whether a micro RNA (miRNA) control mechanism may contribute to the regulation of integrin-dependent processes is not known. However, for the lin41/wech/dappeld gene family, miRNA control is well studied. In C. elegans, the lin41 gene is a target of the let-7 miRNA.Citation17 Drosophila wech/dappled also contains three let-7 regulatory sites present in the 3′-region of its transcript,Citation16 and it has been suggested that the strict regulation of wech/dappled mRNA levels by let-7 miRNAs may be crucial for normal eye development. Reciprocal expression of the mouse Wech ortholog TRIM71/Lin-41 and the microRNAs let-7 and mir-125 has been observed during mouse embryogenesisCitation25 and computational analysis indicates that the let-7 regulatory binding sites are conserved in the mammalian wech orthologs.Citation26 This suggests that also mammalian Wech expression may be regulated by miRNAs which may cause a discrepancy between mRNA and protein expression patterns.
Another significant feature of Wech proteins is their C-terminal NHL domains.Citation11,Citation13 In Drosophila, NHL domains are only found in Wech and three additional proteins: the Drosophila tumor suppressor proteins BratCitation14 and Mei-P26Citation15 and the newly identified ABBA protein which is also expressed in muscle tissue.Citation16 Mutations in brat cause brain tumorsCitation14,Citation27 and mei-P26 mutations lead to ovarian tumors.Citation15 It has recently been shown that Brat acts as a growth regulator and inhibits self-renewal in one of the two daughter cells of neural stem cells by posttranscriptionally inhibiting dMyc and thereby controlling ribosome biogenesis and protein translation.Citation27 Interestingly, the Drosophila NHL-domain protein Brat is also annotated as being regulated by the miRNA let-7 (miRBase, Sanger Institute). The inactivation of mei-P26 causes overproliferation of germline stem cells, most likely due to the lack of proliferation control.Citation15 The molecular characterization of brat mutations suggests that the NHL domain carries the tumor suppressor function of BratCitation14 and that this domain is involved in regulating proliferation of stem cells. Functions of Brat and Mei-P26 in integrin-dependent adhesion or vice versa, of Wech in growth regulation of stem cells, are not yet known. However, it is of note that Wech is expressed both in the female germline and in the larval nervous system (Löer and Hoch, unpublished results). Hence, it will be interesting to determine whether Wech interacts with other NHL-domain proteins and if Wech has additional functions beyond integrin signalling in the regulation of cell growth and differentiation.
Figures and Tables
Figure 1 Wech is required for integrin-dependent muscle attachment in the Drosophila embryo. (A) muscle attachment pattern of Drosophila wild type embryos shown by actin staining. (B) in wech mutants muscle fail to attach to the body wall and ball up. (C) model of Wech function in integrin-dependent muscle attachment in the Drosophila embryo; Wech is associated with Talin and ILK in the adaptor complex which also contains Tensin and PINCH.
References
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673 - 687
- Bökel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell 2002; 3:311 - 321
- Volk T. Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet 1999; 15:448 - 453
- Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin α7 causes a novel form of muscular dystrophy. Nat Genet 1997; 17:318 - 323
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol 2007; 19:43 - 50
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol 2007; 9:858 - 867
- Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RAH, Fristrom JW. Talin is essential for integrin function in Drosophila. Dev Cell 2002; 3:569 - 579
- Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol 2001; 152:1007 - 1018
- Clark KA, McGrail M, Beckerle MC. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 2003; 130:2611 - 2621
- Torgler CN, Narasimha M, Knox AL, Zervas CG, Vernon MC, Brown NH. Tensin stabilizes integrin adhesive contacts in Drosophila. Dev Cell 2004; 6:357 - 369
- Löer B, Bauer R, Bornheim R, Grell J, Kremmer E, Kolanus W, Hoch M. The NHL-domain protein Wech is crucial for the integrin-cytoskeleton link. Nat Cell Biol 2008; 10:422 - 428
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of “single protein Ring finger” E3 ubiquitin ligases. Bioessays 2005; 27:1147 - 1157
- Slack FJ, Ruvkun G. A novel repeat domain that is often associated with RING finger and B-box motifs. Trends Biochem Sci 1998; 23:474 - 475
- Arama E, Dickman D, Kimchie Z, Shearn A, Lev Z. Mutations in the beta-propeller domain of the Drosophila brain tumor (brat) protein induce neoplasm in the larval brain. Oncogene 2000; 19:3706 - 3716
- Page SL, McKim KS, Deneen B, Van Hook TL, Hawley RS. Genetic studies of mei-P26 reveal a link between the processes that control germ cell proliferation in both sexes and those that control meiotic exchange in Drosophila. Genetics 2000; 155:1757 - 1772
- O'Farrell F, Esfahani SS, Engström Y, Kylsten P. Regulation of the Drosophila lin-41 homologue dappled by let-7 reveals conservation of a regulatory mechanism within the LIN-41 subclade. Dev Dyn 2008; 237:196 - 208
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell 2000; 5:659 - 669
- Leptin M, Bogaert T, Lehmann R, Wilcox M. The function of PS integrins during Drosophila embryogenesis. Cell 1989; 56:401 - 408
- Roote CE, Zusman S. Functions for PS integrins in tissue adhesion, migration and shape changes during early embryonic development in Drosophila. Dev Biol 1995; 169:322 - 336
- Walsh EP, Brown NH. A screen to identify Drosophila genes required for integrin-mediated adhesion. Genetics 1998; 150:791 - 805
- Brower DL, Jaffe SM. Requirement for integrins during Drosophila wing development. Nature 1989; 342:285 - 287
- Delon I, Brown NH. Cell-matrix adhesion: the wech connection. Curr Biol 2008; 18:389 - 391
- Kanamoto T, Terada K, Yoshikawa H, Furukawa T. Cloning and regulation of the vertebrate homologue of lin-41 that functions as a heterochronic gene in Caenorhabditis elegans. Dev Dyn 2006; 235:1142 - 1149
- Lancman JJ, Caruccio NC, Harfe BD, Pasquinelli AE, Schageman JJ, Pertsemlidis A, Fallon JF. Analysis of the regulation of lin-41 during chick and mouse limb development. Dev Dyn 2005; 234:948 - 960
- Schulman BRM, Esquela-Kerscher A, Slack FJ. Reciprocal expression of lin-41 and the microRNAs let-7 and mir-125 during mouse embryogenesis. Dev Dyn 2005; 234:1046 - 1054
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000; 408:86 - 89
- Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 2006; 124:1241 - 1253