Abstract
Adult subventricular zone neurogenesis is an important process in most mammals. However, whether it persists in humans is highly debated. Recent work by Sanai and colleagues provides a major step in settling this debate. Using histological approaches, they demonstrated an active subventricular zone with limited neurogenesis in humans as well as discovered a new migratory route.
Introduction
Neurogenesis, the process by which new neurons are generated, continues after birth and into adulthood in the brains of many mammals. Increasing evidence supports its crucial role in brain functions including learning and memory.Citation1 Altman’s pioneering workCitation2,Citation3 provided the first evidence of adult mammalian neurogenesis restricted to two neurogenic brain areas: the hippocampusCitation2 and the subventricular zone (SVZ)Citation3 of the lateral ventricles. Although adult neurogenesis was also found in humans in the dentate gyrus,Citation4 until recently its putative extension to the human SVZ remained in question. Newly produced immature nerve cells (neuroblasts) in the SVZ reach the olfactory bulb (OB) by undergoing tangential chain migration along the rostral migratory stream (RMS) to the OB, where they eventually differentiate to become new OB neurons.Citation1 While Bédard and ParentCitation5 reported a comparable process in adult human SVZ to that of rodents, Sanai and colleaguesCitation6 did not find such evidence. Instead, they reported a markedly different organization. Though they found a specific ribbon of astrocytes that behave as multipotent neuroblasts in vitro, they observed only a few proliferating and migrating neuroblasts in vivo with no evidence of chains of migration, neither in the SVZ nor in the RMS to the OB.Citation6 In contrast, in 2007, Curtis et al.Citation7 reported a massive proliferation in adult human SVZ and intense migration via a RMS along a putative lateral ventricular extension found to connect the human SVZ to the OB. Such possible RMS existence in humans was immediately contested by Sanai et al.,Citation8 who raised concerns about technical demonstration insufficiency. More recently, an immunohistochemical study on the precise cytoarchitecture of human migratory stream supported original findings from Curtis and collaborators.Citation9
The recent work of Sanai et al.Citation10 provides the first developmental study of the human SVZ. The authors aimed at highlighting the organization, cytoarchitecture and ultrastructure of the SVZ of the anterior horn of the lateral ventricle, using serial sections prepared from a remarkable collection of human brain tissue gathered at different ages. Simultaneously, they intended to evaluate the extent and target(s) of migration from neonatal to adult stages.
Key Results and Related Methods
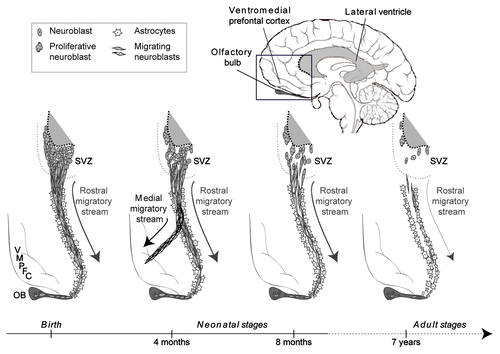
In the recent article published in Nature in 2011, Sanai et al.Citation10 performed fluorescent immunohistochemistry using a wide range of specific markers (for cell proliferation, migration and cell lineages) and in situ hybridization using postmortem brain tissue from humans aged from birth to 84 years. Using indirect immunoenzyme detection for the most specific markers of cell proliferation, migration and cell types (), they characterized SVZ organization across the human lifespan and revealed a dynamic RMS from the SVZ to the OB in neonates. Importantly, quantification analyses showed a drastic decrease in proliferating and migrating neuroblasts from birth to 18 mo leaving only very few in adults. Ultrastructural observations on section and whole mount preparations revealed the organization of RMS as uninterrupted chains of migrating neuroblasts surrounded by glia in neonates. In older children, migrating cells appear individually or in pairs without forming chains. Unexpectedly, serial coronal reconstructions of the frontal lobe revealed a medial migration stream (MMS) of neuroblasts branching off the RMS toward a particular cortical area: the ventromedial prefrontal cortex (VMPFC) (). This MMS was observed only in humans aged 4–6 mo.
Figure 1. Postnatal neurogenesis in the human SVZ. From birth through neonatal stages, the human SVZ is densely populated by neuroblasts (expressing doublecortin and β-III tubulin immature neuronal markers); some of them are proliferative (expressing Ki67 marker). Neuroblasts derived from the SVZ have a migratory phenotype (unipolar and bipolar elongated cells expressing polysialylated neural cell adhesion molecule) and move rostrally to the OB in tangential oriented cellular chains via the RMS. From 4 to 6 mo old, some migrating neuroblasts (expressing interneuron markers calretinin and tyrosine hydroxylase) take a second route through the MMS targeting the VMPFC. In adult stages, the SVZ is depleted of most of its neuroblasts, leaving behind only a few. The remaining migrating neuroblasts appear alone or in pairs along the expected route of the RMS, without forming chains. An astrocyte “ribbon” (expressing glial fibrillary acidic protein) which forms a matrix around the chains of migrating neuroblasts is also represented here in a simplified diagram. Those astrocytes might act as a physical guide thereby influencing neuronal migration. The presence of astrocytes along the MMS is not documented and requires further studies.
Discussion
Together the results indicate that the neonate human SVZ and RMS contain an extensive corridor of migrating neuroblasts before 18 mo of age, which is drastically reduced in older children and adults. Unexpectedly, during this window of neurogenesis, a novel migratory stream, the MMS, was found to deliver SVZ neuroblasts to the prefrontal cortex (). Interestingly, this MMS is not observed in mice, suggesting that it might be a trait acquired during evolution. However, similar chains of neuroblasts reaching the frontal cortex from the RMS were found in postpuberal rabbits.Citation11 The presented findings settle conflicting reportsCitation6,Citation7 by providing compelling evidence of a re-organization of the SVZ neurogenic site in early childhood. This is consistent with recent data, which showed an active RMS in the human SVZ at fetal stages while only a few neuroblasts in the adult.Citation12 This leads to the immediate questions of why and how this restructuring occurs. While the issue of its adaptive significance remains to be explained, one hypothesis for this discovery is that neurogenesis in the adult human SVZ may exist at a low rate as the requirement for new neurons may be relatively low, but remains active in order to be quickly induced following CNS insults for self-repair. Further understanding the guidance cues that regulate neuroblasts migrationCitation1,Citation13 are of exceptional importance to develop new cell therapy strategies, all the more that RMS has recently been characterized in large animals to that purpose.Citation14 Finally, the interesting discovery of the time-limited period of MMS specifically targeting the VMPFC suggests that newly arriving neurons may play a role in cognitive tasks and emotional processes, especially considering that these neurons were found to be dopaminergic.Citation15 It may be time to re-explore the role and interactions of the VMPFC during this developmental time window. Future studies of MMS are likely to shed more light on the understanding of some of the neurodevelopmental brain disorders affecting cognition such as schizophrenia and attention deficit disorder.
| Abbreviations: | ||
| CNS | = | central nervous system |
| MMS | = | medial migratory stream |
| OB | = | olfactory bulb |
| RMS | = | rostral migratory stream |
| SVZ | = | subventricular zone |
| VMPFC | = | ventromedial prefrontal cortex |
Acknowledgments
We thank Dr. Dominique Bagnard for his guidance and for his thoughtful comments. The Joint Master in Neuroscience is supported by NEUREX (www.neurex.org).
References
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008; 132:645 - 60; http://dx.doi.org/10.1016/j.cell.2008.01.033; PMID: 18295581
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec 1963; 145:573 - 91; http://dx.doi.org/10.1002/ar.1091450409; PMID: 14012334
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 1969; 137:433 - 57; http://dx.doi.org/10.1002/cne.901370404; PMID: 5361244
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4:1313 - 7; http://dx.doi.org/10.1038/3305; PMID: 9809557
- Bédard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res Dev Brain Res 2004; 151:159 - 68; http://dx.doi.org/10.1016/j.devbrainres.2004.03.021; PMID: 15246702
- Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004; 427:740 - 4; http://dx.doi.org/10.1038/nature02301; PMID: 14973487
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007; 315:1243 - 9; http://dx.doi.org/10.1126/science.1136281; PMID: 17303719
- Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science 2007; 318:393 - , author reply 393; http://dx.doi.org/10.1126/science.1145011; PMID: 17947566
- Kam M, Curtis MA, McGlashan SR, Connor B, Nannmark U, Faull RL. The cellular composition and morphological organization of the rostral migratory stream in the adult human brain. J Chem Neuroanat 2009; 37:196 - 205; http://dx.doi.org/10.1016/j.jchemneu.2008.12.009; PMID: 19159677
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011; 478:382 - 6; http://dx.doi.org/10.1038/nature10487; PMID: 21964341
- Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol 2006; 498:491 - 507; http://dx.doi.org/10.1002/cne.21043; PMID: 16874818
- Wang C, Liu F, Liu YY, Zhao CH, You Y, Wang L, et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res 2011; 21:1534 - 50; http://dx.doi.org/10.1038/cr.2011.83; PMID: 21577236
- Kahle MP, Bix GJ. Structural and chemical influences on neuronal migration in the adult rostral migratory stream. J Cell Sci Ther 2012; S3:002
- Malik SZ, Lewis M, Isaacs A, Haskins M, Van Winkle T, Vite CH, et al. Identification of the rostral migratory stream in the canine and feline brain. PLoS One 2012; 7:e36016; http://dx.doi.org/10.1371/journal.pone.0036016; PMID: 22606243
- Mendez MF. The neurobiology of moral behavior: review and neuropsychiatric implications. CNS Spectr 2009; 14:608 - 20; PMID: 20173686