Abstract
Cell migration can be principally viewed as a chain of well-orchestrated morphological events that lead to dynamic reshaping of the cell body. However, behind the scene of such a “morphological theater” there are very complex, interrelated molecular and physiological processes that drive the cell movement. Among them, ion transport and pH regulation play a key role, with carbonic anhydrase IX (CA IX) emerging as one of the important “molecular actors.” CA IX is a highly active cell surface enzyme expressed in a broad range of solid tumors in response to hypoxia and explored as a clinically useful biomarker of hypoxia and as a therapeutic target. Its biological role is to protect tumor cells from hypoxia and acidosis in the tumor microenvironment. The study published recently by our group showed that CA IX actively contributes to cell migration and invasion. For the first time, we demonstrated CA IX accumulation in lamellipodia of migrating cells and its direct in situ interaction with bicarbonate transporters. Our findings indicate that tumor cells need CA IX not only as a pro-survival factor in hypoxia and acidosis, but also as a pro-migratory component of the cellular apparatus driving epithelial-mesenchymal transition.
Introduction
Cell migration is a fundamental biological phenomenon that underlies tumor invasion and metastasis. Cancer cells can move either individually or collectively, exploiting similar principles of locomotion. In both cases, the migratory cycle consists of cell polarization and formation of a leading edge (i.e., extension of lamellipodium), attachment of the leading edge to the substrate, proteolytic degradation of extracellular matrix, forward translocation of the cell body, release of adhesions and retraction of the cell rear.Citation1 All steps are accompanied by huge changes in the actin cytoskeleton associated with dynamic modulation and/or redistribution of the functionally relevant molecules and generation of polarized intracellular as well as pericellular gradients of ions.Citation2,Citation3
Migration is usually triggered by signals arriving from the extracellular milieu in the form of pro-migratory growth factors (e.g., HGF, PDGF, VEGF and TGF-β), which are transmitted through the corresponding transmembrane receptors (c-Met, PDGFR, VEGFR and TGFβR) to intracellular signal transduction pathways (e.g., those governed by PI3K, MAPK, PKA and others). This leads to changes in the transcriptional profile and activation of the effector functions required for the execution of the migration program.Citation4
However, tumor cells can also start moving in response to physiological stresses present in the tumor microenvironment, particularly to hypoxia and acidosis.Citation5,Citation6 Hypoxia develops in growing tumor tissues due to irregular and defective vasculature that limits supply of oxygen below its consumption. Insufficiently oxygenated cells react through stabilization of an oxygen-sensitive α subunit of the HIF transcription factor, which forms a heterodimer with a constitutive HIF β subunit and activates the expression of a large array of genes coding for proteins involved in cellular adaptation to hypoxic stress. In addition to components of metabolic reprogramming to glycolysis, factors stimulating angiogenesis and other molecules, HIF upregulates many proteins involved in cell migration and invasion that drive epithelial-mesenchymal transition. These include repressors of intercellular contacts (Snail and Slug), regulators of adhesion and cytoskeletal rearrangement (FAK), pro-migratory growth factors and their receptors (VEGF/VEGFR and HGF/c-MET), cell surface proteinases (TACE/ADAM17), intracellular signal transducers (PKA), ion and water transporters and associated enzymes (MCT4, CA IX, AQP), etc.Citation5
Moreover, as a consequence of the metabolic shift to glycolysis, hypoxic cells generate pericellular acidosis, which further supports migration through the functional activation of proteolytic enzymes that degrade extracellular matrix and release membrane-bound growth factors. Acidosis also contributes to activation of intracellular signaling pathways as well as to stimulation of many constituents of the pH regulating machinery, particularly those acting at the leading edge of the migrating cell.Citation6 Thus, hypoxia and acidosis reinforce the growth factor-induced signaling that regulates cell migration. But, where is the role for the carbonic anhydrase IX in this complex picture?
Carbonic Anhydrase IX: More than a Simple Enzyme
CA IX belongs to the α carbonic anhydrase family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide to bicarbonate ions and protons. This simple reaction is essential for many biological processes, which require acid-base balance and depend on spatially and temporally regulated ion transport in various subcellular compartments and across the plasma membrane. There are 15 human CA isoforms out of which three are inactive and 12 range in activity from weak to very strong. Most of these isoenzymes are expressed in differentiated cells and fulfill specialized roles in various tissues and organs, especially in those, which are metabolically highly active, such as the brain, kidney, stomach, pancreas etc. The very efficient cytoplasmic CA II is traditionally perceived as the main enzyme facilitating breathing and production of various body fluids, but there are also other physiologically relevant isoenzymes, such as CA IV-VII and XII-XIV. Their abnormal expression has been implicated in several pathologic situations, including glaucoma, osteopetrosis, edema, kidney failure, etc.Citation7
Although growing tumors are characterized by abnormal ion transport fluxes and disturbed pH gradients, attempts to associate CAs with cancer generally failed until the identification of the transmembrane CA IX isoform in the early nineties.Citation8 Indeed, CA IX is the only isoenzyme, which is predominantly associated with tumors and only rarely expressed in healthy tissues (namely in the stomach mucosa and intestinal crypts). CA IX is broadly distributed in a range of solid tumors and its presence in tumor cells is often associated with an aggressive tumor phenotype. This is due to its strong regulation by hypoxia at several levels.Citation9
Hypoxia: A Major Regulating Factor of CA IX
Transcriptional activation of CA IX depends on HIF-1 that binds to the core promoter immediately upstream of the transcription start site and is thus the principal transcriptional regulator of the CA9 gene.Citation10 This is reflected by the typical regional expression pattern of CA IX throughout a relatively broad perinecrotic zone in most of the hypoxic tumors and by the diffuse expression pattern in renal carcinomas with constitutive activation of HIF following genetic inactivation of its negative regulator pVHL.Citation10 In the promoter, HIF-1 cooperates with the neighboring SP1 transcription factor, which is important especially at low HIF-1 levels, e.g., in highly dense cells.Citation11 Transcription of the CA9 gene is then further modulated by acidosis and by activation of oncogenic pathways.Citation12,Citation13
Hypoxia also promotes correct splicing of the CA9 mRNA, resulting in the full-length transcript, whereas the alternatively spliced transcript lacking exons 8 and 9 encodes a protein with perturbed localization and function. This splice variant is constitutively present at low level in normoxia and in normal tissues.Citation14
In addition, hypoxia activates CA IX at the functional level. This occurs in consequence of the hypoxia-induced increase in cAMP levels and activation of protein kinase A, which phosphorylates Thr443 in the intracellular tail of CA IX. Phosphorylation of Thr443 then mediates inside-out signaling to the extracellular catalytic domain that leads to activation of the CA IX catalytic performance.Citation15
Last, but not least, hypoxia induces shedding of the ectodomain (ECD) of CA IX, which otherwise is a fairly stable protein having a half life of about 40 h in re-oxygenated cells.Citation16 ECD cleavage is executed by TACE/ADAM17 that is itself regulated by severe hypoxia.Citation17,Citation18
It cannot be excluded that hypoxia affects also additional steps in CA IX expression and functioning, but these remain to be elucidated in future investigations.
The Role of CA IX in Hypoxic Tumor Cells
So why then do tumors need CA IX in such an orderly regulated manner? For tumor cells, hypoxia is a considerable physiological stress imposing selective pressure either to adapt, or to escape or to die. Adaptation mechanisms include among others the capacity to resist acidosis resulting from a more or less extensive metabolic shift from oxidative phosphorylation to anaerobic glycolysis that often remains preserved even in re-oxygenated cells (well-known as Warburg effect).Citation19 Albeit glycolysis generates less energy in the form of ATP than oxidative phosphorylation, it is very important for tumor cells, since it supports production of biomass that is needed for their proliferation.Citation20 Main metabolic output of glycolysis is lactic acid, but the oncogenic metabolism also generates excess of protons and CO2.Citation21 In order to preserve neutral or slightly alkaline intracellular pH (pHi), which is imperative for efficient biosynthetic reactions and cell survival, cells developed transport mechanisms that fall into two principal paths—export of lactate and protons, and import of bicarbonate. Export can be mediated by several types of transmembrane ion transporters, such as monocarboxylate transporter (MCT4) and Na+/H+ exchanger 1 (NHE1), which are also influenced by hypoxia and acidosis.Citation4,Citation22 NHE1 is important for the regulation of both pHe and pHi in tumors and contributes to the production and maintenance of a reverse proton gradient.Citation23 Nevertheless, recent studies indicate that acid extrusion by NHE1 represents a dynamic response to acid load, which varies in a cell-specific manner and in most cells with high normoxic activity it is rather inhibited by hypoxia.Citation24
Thus, in certain situations (such as chronic hypoxia), acid extrusion mechanisms are insufficient to stabilize resting pHi at a mildly alkaline level conducive for cell survival and growth. Therefore, the cells utilize also bicarbonate import by a family of bicarbonate transporters, including Na+-coupled HCO3− co-transporters (e.g., NBCe1) and Cl−/HCO3− anion exchangers (e.g., AE2). Bicarbonate transporters are widely distributed, expressed in diverse isoforms and splice variants, and play important roles in maintaining pHi as well as contributing to cell volume control.Citation25 Functional cooperation between NHE1 and AE2 is evident in NHE1-null mice, in which salivary exocrine cells induce Cl−/HCO3− exchanger activity to compensate for NHE1-deficiency and rescue pHi regulation.Citation26 Furthermore, experiments with 3D tumor spheroids show that pHi recovery from intracellular acidification in the hypoxic core cells is slower upon inhibition of bicarbonate transporters than upon inhibition of NHE, supporting the idea that bicarbonate transport is of principal importance for coping with intracellular acidosis.Citation27 In line with this view, NaHCO3 therapy of xenografted mice leads to reduced intratumoral acidity, inhibition of spontaneous metastases and increased survival of animals.Citation28
However, pericellular lactate, protons and CO2, which accumulate in the tumor microenvironment due to hypoxia and/or oncogenic metabolism, create an acidic extracellular milieu that does not permit spontaneous formation of bicarbonate ions. This shortage of substrate for bicarbonate import offers a role for CA IX enzyme activity, which is based on a bicarbonate metabolon concept proven initially for other carbonic anhydrase isoforms (such as CA II and CA IV).Citation29-Citation31 In line with this concept, CA IX uses its extracellular active site to catalyze CO2 hydration in a close spatial and functional cooperation with bicarbonate transporters.Citation32 The catalysis leads to production of bicarbonate ions that are directly delivered to bicarbonate transporters (e.g., NBCe1 and AE2) that, in turn, bring them into the cytosol where they can take up protons to generate CO2. The CO2 can leave the cell by diffusion and further acidifies the pericellular milieu. The same CO2 hydration reaction catalyzed by CA IX generates also protons that remain at the outer side of the plasma membrane and further feed acidosis.Citation33 This model is supported by experimental evidence for the in vitro interaction between the extracellular catalytic domain of CA IX and AE2 as well as between CA IX and NBCe1 (namely its extracellular loop 4) and also by the demonstration that CA IX accelerates bicarbonate flux through these transporters.Citation32,Citation34 Moreover, CA IX can significantly contribute to pHi recovery from intracellular acidosis in the hypoxic cores of 3D spheroids and to regulation of radial pHe and pHi gradients inside the spheroids.Citation35
From the angle of the maintenance of neutral/slightly alkaline pHi, CA IX can be viewed as a part of the machinery that protects tumor cells from acidic metabolism and thereby confers survival advantage.Citation35,Citation36 Nevertheless, from the angle of generating acidic pHe, CA IX can be viewed as an active player in the pro-metastatic cascade, because extracellular acidosis induces cell migration and activates proteases degrading the extracellular matrix and thereby supports invasion of tumor cells into the surrounding tissue. These initial events then enable tumor cells to enter circulation and eventually establish metastases.Citation37-Citation39
CA IX as a Constituent of the Cell Migration Apparatus
As mentioned above, cell migration is a complex phenomenon that depends, among other processes, on the establishment of correct pH gradients along the longitudinal cell axis—with acidic pHe and alkaline pHi at the cell front, and alkaline pHe and acidic pHi at the rear end.Citation2,Citation3,Citation40 The establishment of the gradient is associated with a re-localization of ion transporters (including NHE1, NBCe1 and AE2) to lamellipodia, where they can accomplish intense ion transport in a context-dependent cooperative manner required for the efficient cell movement.Citation41,Citation42 Molecular and pharmacologic studies show that loss or inhibition of these transporters can have pronounced effects on the cell migration. Inhibition of NHE1 in melanoma cells leads to a rapid decrease of their migration rate. On the other hand, the cells with intact NHE1 activity can migrate approximately two times faster in bicarbonate-buffered medium than in bicarbonate-free solution suggesting that combination of both HCO3− and H+ transports is needed for the full migration capability of melanoma cells.Citation43 In view of the recent knowledge of the mechanisms and role of bicarbonate ions in pH regulation, this data clearly supports the cooperative action of NHE1 with bicarbonate transporters to facilitate movement of cells.
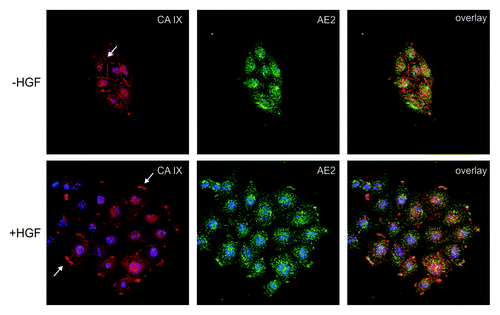
CA IX follows the same re-localization route to protruding cell membranes to occupy a position nearby the bicarbonate transporters (see ). Using a proximity ligation assay that allows for the detection of protein-protein interactions in natural in situ context, we were able to show that CA IX closely communicates with AE2 and NBCe1 in hypoxic tumor cells stimulated to migration by HGF. This was the first direct evidence supporting a somewhat controversial concept of the spatially coordinated bicarbonate transport metabolon operating in a biologically meaningful situation.Citation44
Figure 1. Co-localization of CA IX with AE2 in SiHa cervical carcinoma cells. The cells were grown in islands and exposed to hypoxia for 48 h. Then they were stimulated to migration with HGF (+ HGF) or left un-stimulated (− HGF), double-stained for CA IX (red) and AE2 (green) and subjected to confocal microscopic analysis. In absence of HGF, CA IX was present in the plasma membrane regions involved in intercellular connections, whereas the contact-free membranes were devoid of this protein. Punctate cytoplasmic staining represents precursor forms of CA IX as well as AE2. In HGF stimulated cells, both CA IX and AE2 underwent re-localization to the newly formed lamellipodia in accordance with their coordinated functional involvement in pH control at the protruding membranes of the migrating cells.
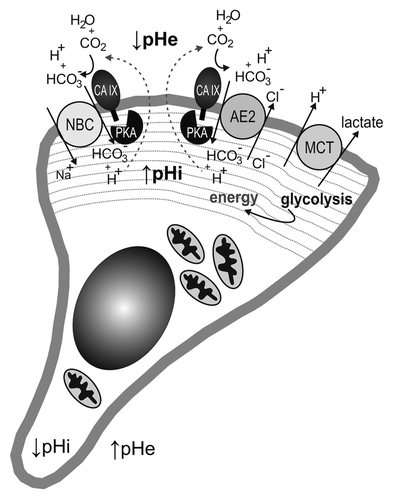
Lamellipodia of moving cells are very interesting cellular compartments from the physiological point of view as they are characterized by intense glycolysis and absence of mitochondria. Indeed, the proteomic analysis of pseudopodia (the structures corresponding to lamellipodia in 3D models) revealed glycolytic enzymes (including GAPDH, enolase, aldolase and phosphofructokinase) as the most abundant group of proteins that facilitate immediate production of energy for cytoskeletal remodeling and protein turnover.Citation45,Citation46 Simultaneously, lamellipodia/pseudopodia are depleted of mitochondrial proteins. This is a metabolic constellation reminiscent of hypoxic cells, so it is possible that lamellipodia are in fact “pseudohypoxic” migration organelles, although there is no proof for this assumption so far. Moreover, this would be compatible with the activation of CA IX function in the bicarbonate transport metabolon which then results in an increased propensity to regulate pH in the frontal cell areas and to stimulate migration both in the presence and absence of HGF.Citation41 In addition, lamellipodia were shown to contain increased cAMP levels that can contribute to the activation of PKA recruited to protrusions during the cell migration.Citation47,Citation48 Moreover, well-known mediators of cell migration (namely WAVE and ezrin) as well as an A-kinase anchoring protein AKAP Lbc contribute to the localization of PKA to the leading edge and formation of the gradient of PKA activity.Citation48-Citation50 Thus, lamellipodial PKA is at the proper position and in the proper physiological context to be able to execute phosphorylation of CA IX and thereby stimulate its catalytic performance coupled with the bicarbonate transport even under normoxic/reoxygenation conditions (). Indeed, phosphorylation of Thr443 is required for the pro-migratory effect of CA IX, because the cells expressing CA IX with inactivating Thr > Ala443 mutation exhibit reduced migration ability.Citation15
Figure 2. Schematic illustration of a migrating cell with a lamellipodium in the protruding front. The lamellipodium (indicated by dashed gray lines) contains bicarbonate metabolon(s) composed of bicarbonate transporter (sodium-bicarbonate co-transporter, NBC, and/or anion exchanger, AE2) cooperating with carbonic anhydrase IX (CA IX). CA IX is activated through the phosphorylation of its C-terminal Thr443 mediated by protein kinase A (PKA), which is also confined to and activated in the lamellipodial compartment. Catalytic conversion of pericellular carbon dioxide by CA IX produces bicarbonate ions that are imported by NBC and/or AE2 in order to increase the intracellular pH (pHi) and at the same time generates extracellular protons that acidify extracellular pH (pHe). The lamellipodium is also characterized by the absence of mitochondria and by a glycolytic metabolism, thus generating energy for the cell remodeling favoring migration and producing lactate that is extruded together with protons to the pericellular space by monocarboxylate transporter (MCT). This leads to a reversed pH gradient at the front membrane, with acidosis outside and slightly alkaline pH inside the lamellipodium.
However, the pro-migratory role of CA IX can be explained also by its ability to interfere with E-cadherin-mediated intercellular adhesion via competitive binding to β catenin in a manner similar to EGFR or MUC-1.Citation51 This can facilitate the dissociation of cells from tissue before they start to migrate and enable faster acquisition of a motile phenotype. Moreover, CA IX suppression by shRNA leads to downregulation of several molecules implicated in the interaction with extracellular matrix and focal adhesion assembly and correspondingly, CA IX-deficient cells display perturbed migration/invasion capacity.Citation52 This is in line with the earlier data showing ability of immobilized CA IX protein to mediate cell attachment to solid support.Citation53
Thus, CA IX possesses several attributes relevant for cell adhesion-migration-invasion and is also able to confer mesenchymal phenotype.Citation44 This is in agreement with its expression in advanced tumors, which often undergo epithelial-mesenchymal transition.Citation54
Open Questions
Although we can now draw the basic contours of the CA IX role in tumor biology, many questions still need to be answered before we get a better understanding of its position in the network of signals driving and regulating cell migration and adaptation to microenvironmental stresses. It would be interesting to see whether the pro-survival role of CA IX can be uncoupled from its pro-migratory role during cancer development, for example in circulating tumor cells that depend on protection from anoikis rather than on protection from acidosis. Furthermore, it remains to be explained whether and how the structural domains determine the functional properties of CA IX, and whether and how CA IX ectodomain shed from the cell surface can impact in a paracrine manner on migratory behavior of recipient cells (and, indeed, which cells can bind this ECD). Also, it would be very attractive to elucidate the crosstalk of CA IX with other cell migration-related pathways induced by growth factors and to clarify the contribution of its post-translational modifications and/or its enzyme activity to this crosstalk. Deeper insight into all these aspects of CA IX involvement in tumor progression including epithelial-mesenchymal transition is required for a better interpretation of its clinical value, and for the development of rational stratification and/or therapeutic strategies based on CA IX targeting.
Finally, from the more general perspective it is worth to note, that the recent investigations of CA IX discussed above clearly point to the importance of hypoxia, which still remains insufficiently explored in the context of cell migration despite commonly accepted view that hypoxic fractions of tumors contain cells with increased migration, invasion and metastatic potential.Citation55 Although literature does show that hypoxia stimulates cell movement and supports wound healing, the role of this key microenvironmental factor in the ion transport-related molecular processes ongoing in lamellipodia of the migrating cells is still abandoned. In fact, the vast majority of studies on mechanisms of ion transport and pH regulation in the migrating cells were performed under normoxic conditions, where metabolic pathways, protein-protein interactions and ion fluxes may well differ from those in hypoxia. Thus, we believe that decoding the biological role of hypoxia-regulated CA IX and its interplay with pH regulators, ion transporters and other relevant molecules in moving hypoxic cells will enable us to get closer to comprehending the complex phenomenon of cell migration as an initial step of the metastatic cascade.
| Abbreviations: | ||
| AE2 | = | anion exchanger 2 |
| AKAP | = | A-kinase anchoring protein |
| AQP | = | aquaporin |
| CA IX | = | carbonic anhydrase IX |
| ECD | = | ectodomain |
| FAK | = | focal adhesion kinase |
| HGF | = | hepatocyte growth factor |
| HIF | = | hypoxia inducible factor |
| MCT | = | monocarboxylate transporter |
| NBC | = | sodium bicarbonate cotransporter |
| NHE | = | Na+/H+ exchanger |
| PKA | = | protein kinase A |
| pVHL | = | von Hippel-Lindau protein |
| TACE/ADAM17 | = | TNFα-converting enzyme/a disintegrin and metalloprotease 17 |
| VEGF | = | vascular endothelial growth factor |
Acknowledgments
The authors’ research is supported by grants from the Slovak Scientific Grant Agency (VEGA 2/0130/11), from the 7th Framework program of EU (Collaborative project METOXIA), from the Research and Development Support Agency (DO7RP-0017-09 and APVV-0658-11) and from the Research and Development Operational Program funded by the ERDF (project ITMS 26240120027). S.P. is the member of the Learned Society of the Slovak Academy of Sciences.
References
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol 2002; 4:E97 - 100; http://dx.doi.org/10.1038/ncb0402-e97; PMID: 11944043
- Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch 2009; 458:981 - 92; http://dx.doi.org/10.1007/s00424-009-0677-8; PMID: 19437033
- Martin C, Pedersen SF, Schwab A, Stock C. Intracellular pH gradients in migrating cells. Am J Physiol Cell Physiol 2011; 300:C490 - 5; http://dx.doi.org/10.1152/ajpcell.00280.2010; PMID: 21148407
- Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res 2010; 8:629 - 42; http://dx.doi.org/10.1158/1541-7786.MCR-10-0139; PMID: 20460404
- Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006; 441:437 - 43; http://dx.doi.org/10.1038/nature04871; PMID: 16724055
- Gillies RJ, Gatenby RA. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metastasis Rev 2007; 26:311 - 7; http://dx.doi.org/10.1007/s10555-007-9065-z; PMID: 17404691
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008; 7:168 - 81; http://dx.doi.org/10.1038/nrd2467; PMID: 18167490
- Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 1994; 9:2877 - 88; PMID: 8084592
- Pastorekova S, Ratcliffe PJ, Pastorek J. Molecular mechanisms of carbonic anhydrase IX-mediated pH regulation under hypoxia. BJU Int 2008; 101:Suppl 4 8 - 15; http://dx.doi.org/10.1111/j.1464-410X.2008.07642.x; PMID: 18430116
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 2000; 60:7075 - 83; PMID: 11156414
- Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastoreková S, Pastorek J, et al. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 α stabilization: a role for phosphatidylinositol 3′-kinase. Cancer Res 2002; 62:4469 - 77; PMID: 12154057
- Ihnatko R, Kubes M, Takacova M, Sedlakova O, Sedlak J, Pastorek J, et al. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int J Oncol 2006; 29:1025 - 33; PMID: 16964400
- Kopacek J, Barathova M, Dequiedt F, Sepelakova J, Kettmann R, Pastorek J, et al. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim Biophys Acta 2005; 1729:41 - 9; http://dx.doi.org/10.1016/j.bbaexp.2005.03.003; PMID: 15833446
- Barathova M, Takacova M, Holotnakova T, Gibadulinova A, Ohradanova A, Zatovicova M, et al. Alternative splicing variant of the hypoxia marker carbonic anhydrase IX expressed independently of hypoxia and tumour phenotype. Br J Cancer 2008; 98:129 - 36; http://dx.doi.org/10.1038/sj.bjc.6604111; PMID: 18026188
- Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, et al. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res 2011; 71:7558 - 67; http://dx.doi.org/10.1158/0008-5472.CAN-11-2520; PMID: 22037869
- Rafajová M, Zatovicová M, Kettmann R, Pastorek J, Pastoreková S. Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol 2004; 24:995 - 1004; PMID: 15010840
- Zatovicova M, Sedlakova O, Svastova E, Ohradanova A, Ciampor F, Arribas J, et al. Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br J Cancer 2005; 93:1267 - 76; http://dx.doi.org/10.1038/sj.bjc.6602861; PMID: 16278664
- Rzymski T, Petry A, Kračun D, Rieß F, Pike L, Harris AL, et al. The unfolded protein response controls induction and activation of ADAM17/TACE by severe hypoxia and ER stress. Oncogene 2012; 31:3621 - 34; http://dx.doi.org/10.1038/onc.2011.522; PMID: 22105359
- Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol 2008; 18:330 - 7; http://dx.doi.org/10.1016/j.semcancer.2008.03.011; PMID: 18455429
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029 - 33; http://dx.doi.org/10.1126/science.1160809; PMID: 19460998
- Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res 2002; 8:1284 - 91; PMID: 11948144
- Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. J Cell Physiol 2011; 226:299 - 308; http://dx.doi.org/10.1002/jcp.22400; PMID: 20857482
- Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 2005; 5:786 - 95; http://dx.doi.org/10.1038/nrc1713; PMID: 16175178
- Hulikova A, Harris AL, Vaughan-Jones RD, Swietach P. Regulation of intracellular pH in cancer cell lines under normoxia and hypoxia. J Cell Physiol 2013; 228:743 - 52; http://dx.doi.org/10.1002/jcp.24221; PMID: 22949268
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch 2004; 447:495 - 509; http://dx.doi.org/10.1007/s00424-003-1180-2; PMID: 14722772
- Gonzalez-Begne M, Nakamoto T, Nguyen HV, Stewart AK, Alper SL, Melvin JE. Enhanced formation of a HCO3- transport metabolon in exocrine cells of Nhe1-/- mice. J Biol Chem 2007; 282:35125 - 32; http://dx.doi.org/10.1074/jbc.M707266200; PMID: 17890222
- Hulikova A, Vaughan-Jones RD, Swietach P. Dual role of CO2/HCO3(-) buffer in the regulation of intracellular pH of three-dimensional tumor growths. J Biol Chem 2011; 286:13815 - 26; http://dx.doi.org/10.1074/jbc.M111.219899; PMID: 21345798
- Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009; 69:2260 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-5575; PMID: 19276390
- Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 2001; 276:47886 - 94; PMID: 11606574
- Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV. J Biol Chem 2002; 277:25239 - 46; http://dx.doi.org/10.1074/jbc.M202562200; PMID: 11994299
- Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3- cotransporter. J Biol Chem 2007; 282:13508 - 21; http://dx.doi.org/10.1074/jbc.M700066200; PMID: 17353189
- Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR. Interactions of transmembrane carbonic anhydrase, CA IX, with bicarbonate transporters. Am J Physiol Cell Physiol 2007; 293:738 - 48; http://dx.doi.org/10.1152/ajpcell.00157.2007
- Svastová E, Hulíková A, Rafajová M, Zat’ovicová M, Gibadulinová A, Casini A, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004; 577:439 - 45; http://dx.doi.org/10.1016/j.febslet.2004.10.043; PMID: 15556624
- Orlowski A, De Giusti VC, Morgan PE, Aiello EA, Alvarez BV. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/HCO3- cotransporter enhances NBCe1-mediated HCO3- influx in the rat heart. Am J Physiol Cell Physiol 2012; 303:C69 - 80; http://dx.doi.org/10.1152/ajpcell.00431.2011; PMID: 22538240
- Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem 2009; 284:20299 - 310; http://dx.doi.org/10.1074/jbc.M109.006478; PMID: 19458084
- Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009; 69:358 - 68; http://dx.doi.org/10.1158/0008-5472.CAN-08-2470; PMID: 19118021
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 2006; 66:5216 - 23; http://dx.doi.org/10.1158/0008-5472.CAN-05-4193; PMID: 16707446
- Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, et al. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis 2008; 25:411 - 25; http://dx.doi.org/10.1007/s10585-008-9145-7; PMID: 18301995
- DeClerck K, Elble RC. The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Front Biosci 2010; 15:213 - 25; http://dx.doi.org/10.2741/3616; PMID: 20036816
- Stock C, Mueller M, Kraehling H, Mally S, Noël J, Eder C, et al. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem 2007; 20:679 - 86; http://dx.doi.org/10.1159/000107550; PMID: 17762194
- Klein M, Seeger P, Schuricht B, Alper SL, Schwab A. Polarization of Na(+)/H(+) and Cl(-)/HCO (3)(-) exchangers in migrating renal epithelial cells. J Gen Physiol 2000; 115:599 - 608; http://dx.doi.org/10.1085/jgp.115.5.599; PMID: 10779317
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol 2001; 280:F739 - 47; PMID: 11292615
- Stüwe L, Müller M, Fabian A, Waning J, Mally S, Noël J, et al. pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J Physiol 2007; 585:351 - 60; http://dx.doi.org/10.1113/jphysiol.2007.145185; PMID: 17916606
- Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem 2012; 287:3392 - 402; http://dx.doi.org/10.1074/jbc.M111.286062; PMID: 22170054
- Jia Z, Barbier L, Stuart H, Amraei M, Pelech S, Dennis JW, et al. Tumor cell pseudopodial protrusions. Localized signaling domains coordinating cytoskeleton remodeling, cell adhesion, glycolysis, RNA translocation, and protein translation. J Biol Chem 2005; 280:30564 - 73; http://dx.doi.org/10.1074/jbc.M501754200; PMID: 15985431
- Nguyen TN, Wang HJ, Zalzal S, Nanci A, Nabi IR. Purification and characterization of beta-actin-rich tumor cell pseudopodia: role of glycolysis. Exp Cell Res 2000; 258:171 - 83; http://dx.doi.org/10.1006/excr.2000.4929; PMID: 10912799
- Lim CJ, Kain KH, Tkachenko E, Goldfinger LE, Gutierrez E, Allen MD, et al. Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol Biol Cell 2008; 19:4930 - 41; http://dx.doi.org/10.1091/mbc.E08-06-0564; PMID: 18784251
- Paulucci-Holthauzen AA, Vergara LA, Bellot LJ, Canton D, Scott JD, O’Connor KL. Spatial distribution of protein kinase A activity during cell migration is mediated by A-kinase anchoring protein AKAP Lbc. J Biol Chem 2009; 284:5956 - 67; http://dx.doi.org/10.1074/jbc.M805606200; PMID: 19106088
- Yamashita H, Ueda K, Kioka N. WAVE2 forms a complex with PKA and is involved in PKA enhancement of membrane protrusions. J Biol Chem 2011; 286:3907 - 14; http://dx.doi.org/10.1074/jbc.M110.145409; PMID: 21119216
- McKenzie AJ, Campbell SL, Howe AK. Protein kinase A activity and anchoring are required for ovarian cancer cell migration and invasion. PLoS One 2011; 6:e26552; http://dx.doi.org/10.1371/journal.pone.0026552; PMID: 22028904
- Svastová E, Zilka N, Zat’ovicová M, Gibadulinová A, Ciampor F, Pastorek J, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res 2003; 290:332 - 45; http://dx.doi.org/10.1016/S0014-4827(03)00351-3; PMID: 14567991
- Radvak P, Repic M, Svastova E, Takacova M, Csaderova L, Strnad H, et al. Suppression of carbonic anhydrase IX leads to aberrant focal adhesion and decreased invasion of tumor cells. Oncol Rep 2013; 29:1147 - 53; http://dx.doi.org/10.3892/or.2013.2226; PMID: 23291973
- Závada J, Závadová Z, Pastorek J, Biesová Z, Jezek J, Velek J. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br J Cancer 2000; 82:1808 - 13; http://dx.doi.org/10.1054/bjoc.2000.1111; PMID: 10839295
- Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle 2004; 3:164 - 7; http://dx.doi.org/10.4161/cc.3.2.618; PMID: 14712082
- Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev 2007; 26:319 - 31; http://dx.doi.org/10.1007/s10555-007-9062-2; PMID: 17458507