Abstract
Magnetic nanoparticles surface-covered with meso-2,3-dimercaptosuccinic acid (MNPs-DMSA) constitute a promising approach for tissue- and cell-targeted delivery of therapeutic drugs in the lung. However, they can also induce a transient transendothelial migration of leukocytes in the organ as a side effect after endovenous administration of MNPs-DMSA. We demonstrated that monocytes/macrophages constitute the main subpopulation of leukocytes involved in this process. Our recent research found that MNPs-DMSA up-regulated the mRNA expression of E-, L- and P-selectin and macrophage-1 antigen, and increased concentration of tumor necrosis factor-α in lung, in a time dependent manner. The critical relevance of the β2 integrin-dependent pathway in leukocyte transmigration elicited by MNPs-DMSA was demonstrated by use of knockout mice. Our work characterizes mechanisms of the pro-inflammatory effects of MNPs-DMSA in the lung, and identifies β2 integrin-targeted interventions as promising strategies to reduce pulmonary side effects of MNPs-DMSA during biomedical applications. In addition, MNPs-DMSA could be used as modulators of lung immune response.
Nanotechnology deals with structures of 100 nm or smaller in at least one dimension and has the potential to create many new materials and devices with a vast range of applications. Materials can be produced that are nanoscale in one dimension (for example, very thin surface coatings), in two dimensions (for example, nanowires and nanotubes) or in all three dimensions (for example, nanoparticles).
Magnetic nanoparticles (MNPs) are a class of nanoparticles that can be manipulated using a magnetic field. MNPs are traditionally ferrite-based materials with the general formula MFe2O4, where M is a doubly charged metal-ion, such as iron, nickel or cobalt. Magnetic fluids (MFs) are colloidal mixtures composed of MNPs suspended in a carrier fluid, usually an organic or inorganic solvent. There is an increasing interest in developing biocompatible MFs for biomedical applicationsCitation1 for instance, for detection of circulating tumor cells,Citation2 contrast agents for magnetic resonance imagingCitation3 and in an experimental cancer treatment called magnetic hyperthermia in which the fact that nanoparticles heat when they are placed in an alternative magnetic field is used.Citation4 Another potential use includes attaching magnetic nanoparticles to drug/gene for targeting purposes.Citation5 In order to be used for medical applications, magnetic nanoparticles are coated with a surfactant to prevent their agglomeration (due to van der Waals and magnetic forces) and allow the association of MNPs surface with different molecules.Citation6,Citation7
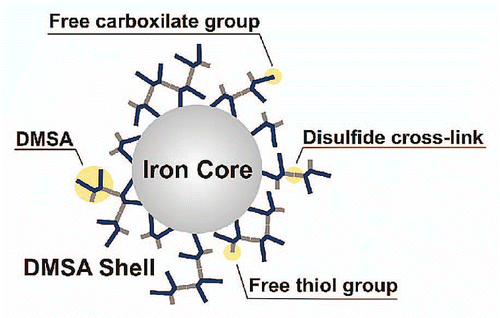
In previous studies, we have shown that MNPs surface-coated with meso-2,3-dimercaptosuccinic acid (MNPs-DMSA) (), with average diameter of about 9 nm, presented preferential distribution in the lung tissue, after intravenous administration in mice.Citation8–Citation10 This target specificity of MNPs-DMSA offers a unique property that may be successfully exploited for the treatment of lung diseases.Citation11 In addition, we reported that the presence of MNPs-DMSA in the lung led to trafficking of leukocytes from blood vessels into pulmonary parenchyma and airspace and that interleukin-1 (IL-1) and interleukin-6 (IL-6) were overexpressed.Citation12 IL-1 acts as a trigger that activates a cascade of cytokine production and induces the production of a wide range of immunomodulatory cytokines.Citation13 IL-6 is among the mediators regulated by IL-1 and is often increased in inflammatory processes in the lung.Citation13 These differential expressions were particularly associated with blood vessels and cells of airway ducts suggesting that they could have some role during the recruitment process of inflammatory cells, as observed in histological analyses. In fact, these cytokines are commonly associated with the activation of cells concerning the expression of adhesion surface proteins.Citation13 This is in agreement with several studies that described the requirement of IL-1α production in rat airways for full polymorphonuclear cell migration in models for immune-complex deposition or inhalation of cement dust, coal dust or diesel exhaust particles.Citation14–Citation16
Cell migration plays a key role in a wide variety of biological phenomena. This process is particularly important for leukocyte function and the inflammatory response. A mechanistic understanding of cellular interactions with synthetic surfaces, particularly in the context of inflammatory and healing responses, has been a major goal of biomaterial science.
Leukocyte trafficking in the lung involves transendothelial migration, migration in tissue interstitium and transepithelial migration. In addition, leukocyte emigration involves regulatory mechanisms including complement activation, cytokine regulation, chemokine production, activation of adhesion molecules and their respective counter receptors. The process is presumably initiated and modulated by the production of early response cytokines such as IL-1 and tumor necrosis factor (TNF) from lung cells, especially from alveolar macrophages, setting the stage for leukocyte migration through endothelium.Citation17 On the other hand, ensuing production of interleukin-10 (IL-10) brings into play powerful anti-inflammatory factors that strongly regulate inflammatory responses, functioning as intrinsic regulators of the lung inflammatory response.Citation18,Citation19
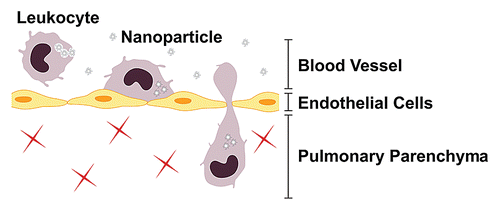
Tissue infiltration by circulating leukocytes is a three-step process involving rolling on the endothelium, attachment to the endothelium and transmigration across the endothelial cells lining blood vessel walls (). Leukocyte migration out of the blood is initiated by leukocyte rolling on the luminal side of the endothelium, as mediated by the low-affinity receptors selectins (E-, L- and P-selectin).Citation20–Citation22 Binding of selectins on leukocytes stimulates “outside-in” signals in these cells, increasing the affinity of the integrin family of receptors (cell surface receptors consisting of an α- and a β-subunit, which are grouped in distinct subfamilies based on β-subunit utilization), which then bind to endothelial cell adhesion molecules such as intercellular adhesion molecule-1 [(ICAM-1)/CD54] and vascular cellular adhesion molecule-1 (VCAM-1). Function-blocking studies have identified the β1 (CD29) and β2 (CD18) integrins as the major players involved in leukocyte adhesion and migration.Citation23 Leukocyte integrin affinity is also rapidly increased by “inside-out” signals from leukocyte chemokine receptors triggered by chemokines displayed on the surface of endothelial cells.Citation24 With an increase in leukocyte integrin receptor affinity, leukocyte rolling is arrested.Citation24
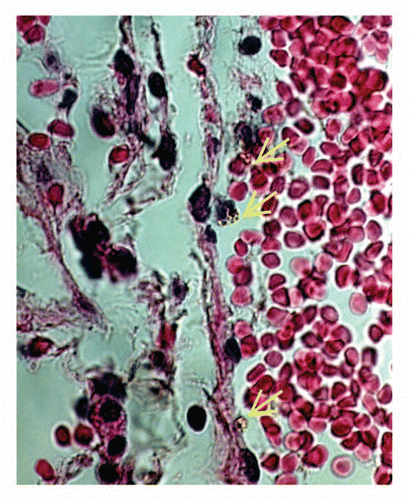
Using immunohistochemistry, we demonstrated that following injection of MF-DMSA, the distribution pattern of E-selectin and members of the β2 integrin subfamily (macrophage-1 antigen, Mac-1; leukocyte function associated antigen-1, LFA-1) was changed in the lung vessels, but not of β1 integrin.Citation10 For L and P selectins no differences were observed between treated and control animals. However, for E-selectin, labeling was found in the endothelium of veins and venules 12 h after MF-DMSA administration, but not in the lung's vascular compartments of the control and 4 h treatment groups.Citation12 Concerning integrins, in the control group, leukocytes labeled with Mac-1 and LFA-1 were found only in post-capillary sites. Four hours after MF-DMSA administration, leukocytes expressing these β2 integrins were also found in capillaries.Citation10 Our findings expand on other studies showing that the capillary network constitutes an important migration site in the lung.Citation25 Thus, the modulation of Mac-1 and LFA-1 expression in leukocytes located inside capillaries supports the importance of these integrins and capillaries for migratory activity in the lung, in this case after MF-DMSA administration. However, we cannot discard the participation of larger vessels in the migration induced by MNPs-DMSA. In fact, some images from our laboratory have showed that this is also a route used by the leukocytes after injection of these nanoparticles ().
It is worth noting that 12 h after MF-DMSA administration, leukocytes labeled with LFA-1 were observed only in post-capillary sites, similar to the control. We speculated that the absence of LFA-1 labeling in capillaries in the period of 12 h after MF-DMSA administration is due to the accentuated decrease of LFA-1 expression levels in the leukocyte over the course of time. In fact, as will be discussed below, we obtained a decrease in the LFA-1 mRNA 12 h after MNPs-DMSA administration. This point of view is in agreement with other studies that demonstrated the distinct contribution of LFA-1 and Mac-1 to transendothelial migration in the lung.Citation26 While both Mac-1 and LFA-1 participate in transendothelial migration at the beginning of the inflammatory process, over time Mac-1 becomes the predominant member of the β2 integrin subfamily mediating migration of leukocytes.Citation26
These results raised several questions related to MNPs-DMSA administration, such as: what is the time profile of leukocyte migration into the airspace? Which is the principal leukocyte subpopulation involved in this process? Is it a fact that the mechanism by which the presence of MNPs-DMSA induces transendothelial migration of leukocytes into the lung is based on their ability to somehow change the expression of cell adhesion molecules on leukocytes and lung vascular endothelial cells? Is β2 or β1 integrin, or both, the main receptor involved in MNPs-DMSA leukocyte-induced migration?
Recently, we uncovered some of these answers including the main adhesion molecules that are involved in this migration. We first determined that the number of leukocytes in the bronchoalveolar lavage fluid reached its peak 12 h after MNPs-DMSA administration, decreasing to normal values in 48–72 h. Cytologic and FACS analysis demonstrated that the main subpopulation of leukocytes involved in this process was monocyte/macrophage.Citation27
It is well known that the reticuloendothelial system, in particular macrophage cells, actively neutralizes and eliminates foreign matter from the body, including nonbiological particles. These and other particulated materials in the lung may lead to lung damage. In fact, transmission electron microscopy analysis clearly demonstrated an uptake of MNPs-DMSA by monocyte/macrophage cells,Citation27 indicating that this may be a mechanism of nanoparticle clearance used by the lung in order to avoid further damage. It is worth noting that an increase in the relative percentage of lymphocytes after MNPs-DMSA administration was also observed. The importance of this finding was not addressed in the paper, but we speculate that it could be important for the control of the inflammatory process initiated by the MNPs-DMSA injection. Failure in control of the inflammatory processes could potentially lead to chronic inflammatory diseases and pulmonary fibrosis. In spite of the fact that we did not determine which was the main source of the production of two different cytokines, one considered pro-inflammatory (TNFα) and the other anti-inflammatory (IL-10), we found an increase in the ratio of IL-10/TNFα cytokine release 12 h after MNPs-DMSA administration. This is clearly a signal that the inflammatory process was being controlled, in agreement with previous reports showing that IL-10 is able to limit the induction of cell adhesion molecules in the lung.Citation28 We presume that lymphocytes are taking part in this process. Further studies are necessary to clarify this point.
The nature of the cells present in the pulmonary tissue parenchyma was not determined in this study. However, these cells were not able to cause tissue damage in the lung. We observed no histological or ultrastructural damage in the lung of animals treated with MNPs-DMSA, indicating that the nanoparticle-induced inflammation is not enough to cause chronic disease, such as pulmonary fibrosis.
We then determined the effect of MNPs-DMSA on mRNA expression of selectins, integrin β1 and integrin β2.Citation27 We found that MNPs-DMSA upregulated the mRNA expression of E-, L- and P-selectin, as well as Mac-1. Further, using knockout mice (deficient in the β2-subunit common to all β2 integrins), we observed that, compared to wild-type mice, the recruitment of leukocytes to the airspace following administration of MNPs-DMSA was completely blocked in the former.Citation27 The fact that transmigration of β2 integrin-deficient monocytes was affected when compared with wild-type monocytes strongly argues in favor of a major contribution by β2 integrins to monocyte trans-epithelial migration in our system, which is additionally supported by the increase of mRNA of β2 integrins, as cited above.
We should remember, however, that the absence of change in LFA-1 and very late antigen-4 (VLA-4) mRNA does not exclude a role for them in leukocyte migration induced by MNPs-DMSA. Integrins are cell adhesion molecules constitutively expressed on the cell surface and also stored within intracellular vesicles.Citation29,Citation30 In addition, transendothelial migration of leukocytes depends not only on the number of integrins on the cell surface but also on the change in conformation of these molecules reflecting their activation.32 Therefore, our results did not exclude the possibility that MNPs-DMSA induce the activation of LFA-1 and VLA-4 constitutively located on the surface of leukocytes or the translocation of these integrins from intracellular vesicles to the plasma membrane. On the other hand, the absence of a significant change in the mRNA expression of VCAM-1, which is the major endothelial cell ligand for VLA-4, can be regarded as an indirect indicator that VLA-4 is not involved in this process.
The fact that an increase in the mRNA of Mac-1 occurred and there is no change in the mRNA levels of VLA-4 (and LFA-1) corroborates the hypothesis that migration of leukocytes induced by MNPs-DMSA is mainly dependent on β2 integrins and not β1 integrins pathway. In addition, we can presume that MAC-1 is the main β2 integrin molecule involved in the process of leukocyte trafficking.
The increased use of nanoparticles in medicine has raised concerns on their ability to gain access to privileged sites in the body. In fact, a study has shown that, in some cases, they can potentially cause damage to tissues located behind cellular barriers. Therefore, it is fundamental to understand the mechanisms underlying interactions between nanoparticles and the body, for their safe and effective use. In the case of MNPs-DMSA, we can use this knowledge for treatment of lung diseases when associated with drugs, as well as for downregulation or upregulation of the local immune system.
One important question still unanswered about the use of magnetic nanoparticles in lung disease treatments is what could be expected if more than one dose is necessary in a short period of time. Recent research of Mejias et al.Citation31 was close to answer this question. In their study the authors injected repeated doses (nine in total) of magnetic nanoparticles stabilized with DMSA, but unfortunately, they did not analyze the lungs, assuming that the particles would be stocked in the liver, spleen and kidney. For these organs, however, the authors did not refer to any observed damage. We believe that the answer to this question is related with several factors such as physical-chemical features of the nanoparticles (size, hydrodynamic radius, etc.) interval between the injections, amount of iron injected, among others. These features are also important for a second open question: what happens if the organ has a preexistent disease? Further studies are necessary to clarify this point. It is important to minimize, in all cases, the amount of injected iron, increasing, when possible, the amount of drug attached to the nanoparticles. The use of magnetic nanoparticles is already a reality as a contrast agent. It is possible that in the future they also can be used as drug delivery carriers.
In resume our work characterizes mechanisms of the pro-inflammatory effects of MNPs-DMSA in the lung and identifies β2 integrin-targeted interventions as promising strategies to reduce pulmonary side effects of MNPs-DMSA during biomedical applications. In addition, MNPs-DMSA could be used as modulators of lung immune response.
Abbreviations
| MNPs | = | magnetic nanoparticles |
| MFs | = | magnetic fluids |
| MNPs-DMSA | = | MNPs surface-coated with meso-2,3-dimercaptosuccinic acid |
| IL-1 | = | interleukin-1 |
| IL-6 | = | interleukin-6 |
| TNF | = | tumor necrosis factor |
| IL-10 | = | interleukin-10 |
| E-, L- and P-selectin | = | selectins |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| VCAM-1 | = | vascular cellular adhesion molecule-1 |
| VLA-4 | = | very late antigen-4 |
Figures and Tables
Acknowledgements
The authors thank the Brazilian agencies MCT/CNPq, FINATEC, FAP-DF and FINEP for financial support and Dr. C.M. McManus for the English correction of this manuscript.
References
- Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 2005; 100:1 - 11
- Galanzha EI, Shashkov EV, Kelly T, Kim JW, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nanotechnol 2009; 4:855 - 860
- Heyn C, Ronald JA, Mackenzie LT, MacDonald IC, Chambers AF, Rutt BK, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med 2006; 55:23 - 29
- Kim DH, Kim KN, Kim KM, Lee YK. Targeting to carcinoma cells with chitosan- and starch-coated magnetic nanoparticles for magnetic hyperthermia. J Biomed Mater Res A 2009; 88:1 - 11
- McCarthy JR, Kelly KA, Sun EY, Weissleder R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomedicine 2007; 2:153 - 167
- Morais PC, Garg VK, Oliveira AC, Azevedo RB, Rabelo D, Lima ECD. Synthesis and characterization of magnetite nanoparticles embedded in copolymer microspheres. Eur Cell Mater 2002; 3:173 - 175
- Freitas MLL, Silva LP, Azevedo RB, Garcia VAP, Lacava LM, Grisolia CK, et al. A double-coated magnetite-based magnetic fluid evaluation by cytometry and genetic tests. J Magn Magn Mat 2002; 252:396 - 398
- Chaves SB, Lacava LM, Lacava ZGM, Silva O, Pelegrini F, Buske N, et al. Light microscopy and magnetic resonance characterization of a DMSA-coated magnetic fluid in mice. IEEE Trans Magn 2002; 38:3231 - 3233
- Garcia MP, Parca RM, Chaves SB, Silva LP, Santos AD, Lacava ZGM, et al. Morphological analysis of mouse lungs after treatment with magnetite-based magnetic fluid stabilized with DMSA. J Magn Magn Mater 2005; 293:277 - 282
- Valois CRA, Nunes ES, Jaeger RG, Lima ECD, Morais PC, Azevedo RB. Expression patterns of cell adhesion molecules in mice's lung after administration of meso-2,3-dimercaptosuccinic acid-coated maghemite nanoparticles. J Nanosci Nanotechnol 2009; 9:2846 - 2855
- Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol 2005; 23:1418 - 1423
- Chaves SB, Silva LP, Lacava ZGM, Morais PC, Azevedo RB. Interleukin-1 and interleukin-6 production in mice's lungs induced by 2, 3 meso-dimercaptosuccinic-coated magnetic nanoparticles. J Appl Phys 2005; 97:1 - 3
- Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res 2010; 11:16 - 30
- Pande M, Flora SJS. Lead induced oxidative damage and its response to combined administration of alpha-lipoic acid and succimers in rats. Toxicology 2002; 177:187 - 196
- Gosset P, Lassalle P, Vanhee D, Wallaert B, Aerts C, Voisin C, et al. Production of tumor-necrosis-factor-alpha and interleukin-6 by human alveolar macrophages exposed in vitro to coal-mine dust. Am J Respir Cell Mol Biol 1991; 5:431 - 436
- van Berlo D, Haberzettl P, Gerloff K, Li H, Scherbart AM, Albrecht C, et al. Investigation of the cytotoxic and proinflammatory effects of cement dusts in rat alveolar macrophages. Chem Res Toxicol 2009; 22:1548 - 1558
- Inoue G. Effect of interleukin-10 (IL-10) on experimental LPS-induced acute lung injury. J Infect Chemother 2000; 6:51 - 60
- Lo SK, Everitt J, Gu J, Malik AB. Tumor necrosis factor mediates experimental pulmonary edema by ICAM-1 and CD18-dependent mechanisms. J Clin Invest 1992; 89:981 - 988
- Mulligan MS, Jones ML, Vaporciyan AA, Howard MC, Ward PA. Protective effects of IL-4 and IL-10 against immune complex-induced lung injury. J Immunol 1993; 151:5666 - 5674
- Liu Y, Shaw SK, Ma S, Yang L, Luscinskas FW, Parkos CA. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J Immunol 2004; 172:7 - 13
- Maus U, Huwe J, Ermert L, Ermert M, Seeger W, Lohmeyer J. Molecular pathways of monocyte emigration into the alveolar air space of Iitact mice. Am J Respir Crit Care Med 2002; 165:95 - 100
- Nourshargh S, Marelli-berg FM. Transmigration through venular walls: a key regulator of leukocyte phenotype and function. Trends Immunol 2005; 26:157 - 165
- Cao C, Lawrence AD, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 2005; 106:3234 - 3241
- Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol 2005; 77:487 - 495
- Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev 2003; 83:309 - 336
- Neelamegham S, Taylor AD, Shankaran H, Smith CW, Simon SI. Shear and time-dependent changes in Mac-1, LFA-1 and ICAM-3 binding regulate neutrophil homotypic adhesion. J Immunol 2000; 164:3798 - 3805
- Valois CRA, Braz JM, Nunes ES, Vinolo MAR, Lima ECD, Curi R, et al. The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via a β2 integrin-dependent pathway. Biomaterials 2010; 31:366 - 374
- Mulligan MS, Jones ML, Vaporciyan AA, Howard MC, Ward PA. Protective effects of IL-4 and IL-10 against immune complex-induced lung injury. J Immunol 1993; 151:5666 - 5674
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673 - 687
- Chan P, Aruffo A. VLA-4 integrin mediates lymphocyte migration on the inducible endothelial cell Ligand VCAM-1 and the extracellular matrix ligand fibronectin. J Biol Chem 1993; 268:24655 - 24664
- Mejías R, Pérez-Yagüe S, Rocal AG, Pérez N, Villanueva A, Cañete M, et al. Liver and brain imaging through dimercaptosuccinic acid-coated iron oxide nanoparticles. Nanomedicine 2010; 5:397 - 408