Abstract
We have delineated TGFβ signaling pathways in the production of osteolytic factors interleukin-8 and interleukin-11 in breast cancer cells with different bone metastases potential. Bone seeking MDA-MB-231(hm) cells expressed higher levels of IL-11, but lower levels of IL-8 compared to MDA-MB-231 cells. MCF-7 cells (mainly osteoblastic) did not express IL-8 or IL-11; MDA-MB-468 cells (weakly metastatic) expressed IL-8, but not IL-11. The up-regulation of IL-11 and IL-8 was associated with the rapid activation of SMAD2/3 and p38 MAPK through the TGFβ/TGFβR system. Analysis of TGFβ receptors indicated that MCF-7 cells do not express TGFβRII, and MDA-MB-468 cells do not express SMAD4. Inactivation of SMAD4 or p38PMAPK gene via RNAi resulted in the inhibition of IL-11 and IL-8 production in MDA-MB-231(hm) cells; and over-expression of SMAD4 gene resulted in IL-11 production in MDA-MB-468 cells. TGFβ-1 induced SMAD3 translocation to the nuclei in MDA-MB-231, MDA-MB-231(hm) as well as in SMAD4 deficient MDA-MB-468, indicating that an alternate non-canonical pathway could be responsible for TGFβ-1 induced cytokine production in MDA-MB-468 cells. Thus, four breast cancer cell lines used in this study show differential expression and up-regulation of the osteolytic factors in response to TGFβ-1 that involves both SMAD pathway, a non-canonical SMAD pathway, as well as p38 MAPK pathways.
Introduction
Despite the recent advances in the detection and treatment options available for breast cancer patients, metastases to the secondary sites, especially the bone is still the primary cause of severe pain and mortality.Citation1 Transforming growth factor beta (TGFβ) is a pleiotropic growth factor eliciting a wide range of effects on cell growth; and has been implicated in playing a significant role in the invasion and subsequent metastases including bone metastases.Citation2–Citation7 It has been suggested that the presence of TGFβ in the bone microenvironment may play a significant role in bone destruction at the site of tumor metastasis by inducing osteolytic cytokines IL-11,Citation8 and IL-8.Citation9 This is particularly significant since the bone matrix acts as a storage site for many factors, including TGFβ⊠ which can be released during osteolysis of the bone.Citation5,Citation10
In this communication we have addressed several important questions related to TGFβ signaling and IL-8 and IL-11 production, in breast cancer cells. Although IL-11 and IL-8 have been proposed to be critical osteolytic factors, their roles in other breast cancers such as estrogen receptor positive MCF-7 cells which tends to form osteoblastic lesions, or MDA-MB-468 cells which are poorly metastatic, are relatively less understood. It is known that TGFβ-1 bound TGFβRII recruits and rapidly phosphorylates TGFβRI, which in turn phosphorylates SMAD2 and SMAD3 proteins or can use pathways such as PI3 Kinase-Akt and p38-MAPK.Citation3,Citation11–Citation13 In this study, we have addressed the possible role of SMAD and p38 MAPK for the production of IL-11 and IL-8 in breast cancer cells. The activated forms of SMAD2 and SMAD3 protein bound to SMAD4 are translocated to the nuclei, where several genes are up or downregulated.Citation14 We have investigated if in cells which lack SMAD4, whether a non-canonical pathway exists whereby the activated SMAD2 and SMAD3 can still be shuttled to the nucleus via a non-canonical pathway in the absence of SMAD4. The results presented indicate multiple TGFβ signaling pathways that are potentially involved in the regulation of IL-11 and IL-8 production in cells with varied bone metastases potential.
Results
Differential upregulation of IL-11 and IL-8 cytokines in response to TGFβ-1.
The levels of osteolysis promoting cytokines IL-11 and IL-8 in the absence and presence of TGFβ-1 in MCF-7, MDA-MB-231, MDA-MB-231 (hm) and MDA-MB-468 breast cancer cell lines were measured over a period of 24 hours. MCF-7 cells do not secrete detectable IL-11 () or IL-8 (). Both MDA-MB-231 and MDA-MB-231 (hm) cells secrete IL-11 and IL-8, though MDA-MB-231 (hm) secretes 3- to 4-folds higher levels of IL-11 as compared to MDA-MB-231 cells. Interestingly, MDA-MB-468 cells secrete only IL-8.
Secreted levels of IL-8 and IL-11 cytokines also varied in the breast cancer cells treated with TGFβ-1 ( and B). While this induction is absent in MCF-7 cells, treatment of MDA-MB-231 and MDA-MB-231 (hm) cells with TGFβ-1 further enhanced the IL-11 production by 2- to 3-fold. The levels of IL-8 cytokine were also increased on stimulation with TGFβ-1 ( and B). However, the fold increase observed in the level of IL-8 cytokine secretion by TGFβ-1 stimulation was not as significant as that seen for IL-11 cytokine.
Involvement of SMADs and p38 MAPK in the downstream effects of TGFβ in breast cancer cells.
We further aimed to dissect the TGFβ-1 signaling pathway involved in the regulation of each cytokine. The signaling pathway downstream of TGFβ-1 through its transmembrane serine/threonine kinase receptors TGFβRI and TGFβRII has been well discerned. SMAD3 was activated by phosphorylation on treatment with 1 ng/mL TGFβ treatment within 60 minutes in MDA-MB-231, MDA-MB-231(hm) and MDA-MB-468 cell lines (). MCF-7 cells have high basal levels of phospho-SMAD3 but do not show any significant upregulation on treatment with TGFβ-1. Similarly, MDA-MB-231, MDA-MB-231(hm) and MDA-MB-468 cell lines also showed an upregulation of phospho-SMAD2 with 1 ng/mL TGFβ-1 (not shown), and p38 MAPK by phosphorylation (). There was no corresponding activation of p38 MAPK in MCF-7 cells, again indicating their unresponsiveness to TGFβ1 ().
To examine if both SMAD4 dependent transcription and p38 MAPK activation pathway could participate in the IL-11 and/or IL-8 induction, we studied the upregulation of these cytokines in the presence and absence of SMAD4 RNAi or p38 MAPK RNAi or control RNAi in MDA-MB-231(hm) as described in materials and methods. The co-incubation of TGFβ-1 with SMAD4 specific RNAi (20 nM) reduced SMAD4 expression () and inhibited the IL-11 and IL-8 levels compared to control RNAi ( and C). The co-incubation of TGFβ-1 with p38 MAPK RNAi (40 nM) reduced the IL-8 levels compared to control RNAi (). These results confirm that IL-11 and IL-8 induction could involve activation of both SMAD and p38 MAPK pathways. The involvement of SMAD4 in mediating IL-11 was further examined using MDA-MB-468 cells overexpressing SMAD4. As shown in , MDA-MB-468 cells transfected with SMAD4 expressing plasmid DNA produced IL-11, which was further increased by TGFβ-1.
Investigating the TGFβ-1 signaling pathway.
To resolve the lack of TGFβ-1 response in MCF-7 cell line, we further aimed to dissect the TGFβ-1 signaling pathway from its origination at the receptors. The expression levels of the two TGFβ receptors-TGFβRI and TGFβRII were examined. Expression of mRNA for both the receptors was confirmed in MDA-MB-231, MDA-MB-231 (hm) and MDA-MB-468. However, MCF-7 cells either lack or have very low copies of mRNA transcripts coding for the TGFβ sequestering TGFβRII receptor (). It is possible that deficiency of the TGFβRII receptor does not allow the presentation of TGFβ ligand and the subsequent activation of its signaling receptor TGFβRI explaining its inability to activate SMAD2/3, p38 MAPK pathways, and IL-11 and IL-8 production in MCF-7 cells.
Typically, upon activation of the TGFβ signaling pathway, the phosphorylated forms of SMAD2 and SMAD3 are then bound by SMAD4 and channeled into the nucleus where they regulate transcription of specific genes. Expression of SMAD4 in all four cell lines was therefore examined by western blot analysis. Three cell lines MDA-MB-231, MDA-MB-231(hm) and MCF-7 showed SMAD4 expression. However, MDA-MB-468 lacks the SMAD4 protein ().
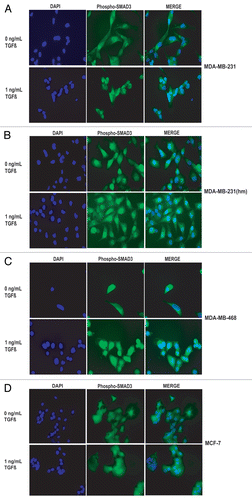
To examine the TGFβ-dependent movement of SMAD to the nuclei, we conducted phospho-SMAD3 immunofluorescent staining of serum starved MDA-MB-231, MDA-MB-231(hm) and MDA-MB-468 cells treated with 1 ng/mL TGFβ-1. In the absence of TGFβ-1 treatment a diffuse staining of phospho-SMAD3 was observed. There was a distinct nuclear localization of phospho-SMAD3 within 60 minutes of treatment with 1 ng/mL TGFβ-1 in both MDA-MB-231 and MDA-MB-231 (hm) cell lines (). Interestingly, the nuclear localization of phospho-SMAD3 was also seen in the SMAD4 deficient MDA-MB-468 cells suggesting the presence of non-canonical pathway for nuclear translocation of phosphorylated forms of SMAD2/3 in absence of SMAD4 protein. In MCF-7 cells, there was some nuclear translocation in the absence of TGFβ-1, however there was no additional nuclear localization of phospho-SMAD3 after treatment with TGFβ-1 (); a phenomenon consistent with higher basal levels of p-SMAD2/3 and lack of TGFβ-1-mediated induction of p-SMAD as described earlier in .
Discussion
Several important findings pertaining to the relationship of TGFβ signaling, IL-11 and IL-8 production in breast cancer cell models with different bone metastases potential have been presented. MCF-7 cells do not seem to express TGFβRII receptor, and are unable to induce TGFβ-dependent SMADs phosphorylation, MAPK phosphorylation and IL-11 or IL-8 induction. It is tempting to speculate that because of lack of TGFβ responsiveness, MCF-7 cells mainly induce osteoblastic bone metastases; TGFβ is known to be a key player in the vicious cycle involved in the osteolytic bone destruction.Citation5,Citation10,Citation15 The argument for the role of TGFβ signaling and of IL-11 in particular, in inducing bone metastases, is strengthened by the noted difference between the MDA-MB-231 and the bone seeking MDA-MB-231(hm). The basal level of IL-11 in the bone seeking MDA-MB-231(hm) is much higher, compared to the MDA-MB-231 cells. However, TGFβ dependent SMAD2 and SMAD3 phosphorylation, p38 MAPK activation and nuclear localization of p-SMAD3 are similar in MDA-MB-231 and MDA-MB-231(hm) cells; some of these similarities between MDA-MB-231 and MDA-MB-231(hm), might reflect the fact that even MDA-MB-231 cells are known to form bone metastasis, albeit at reduced rate.Citation16 Based on this, we conclude that TGFβ-dependent IL-11 induction is critical to provide bone metastases phenotype. This conclusion is reinforced by MDA-MB-468 cell data. This cell line does not express SMAD4 and does not produce IL-11; but has capacity to express IL-8 cytokine. Given that this cell model does have a non-canonical pathway for SMAD2/SMAD3 activation; and p38 MAPK activation, and yet is only weekly metastatic, again argues for the role of SMAD4-dependent IL-11 production for inducing osteolysis.
In summary, the work presented here using multiple breast tumor cell models suggests that the induction of the osteolytic cytokines IL-11 and IL-8, is controlled by divergent signaling pathways downstream of TGFβ-1 stimulation of cells. Given an intense interest in developing anticancer strategies by targeting TGFβ pathways, these downstream signals could also offer therapeutic targets for the treatment of bone metastases.Citation3,Citation17–Citation21
Materials and Methods
Cell culture.
MCF-7, MDA-MB-231 and MDA-MB-468, were obtained from ATCC (Manassas, VA). Bone-seeking MDA-MB-231 (hm) was a kind gift from Dr. Theresa Guise (Indiana University). All cells were grown as described previously in reference Citation19, Citation20 and Citation22.
SMAD2/3 and p38 MAPK phosphorylation studies, SMAD4 expression.
Cells were plated in 6-well plates at a density of 4 × 105 cells/well. After one day in culture, cells were starved for 24 hours in serum free media. The cells were washed with serum free media before treatment with TGFβ-1 (Sigma Life Science) for one hour, and cell lysates subjected to western blot analyses using published methods.Citation19,Citation22 Membranes were treated with phospho-SMAD2, phospho-SMAD3, SMAD2/3, phospho-p38 MAPK or p38 MAPK antibodies (Cell Signaling) followed by horseradish peroxidase-conjugated bovine anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology). The blots were visualized by enhanced chemiluminescence substrate (Amersham Biosciences). For examining, SMAD4 expression, cell lystates were subjected to western blots and probed with anti-SMAD4 antibodies (Cell Signaling).
Enzyme linked immunosorbent assays.
Cells were plated at a density of 2 × 105 cells/well of 6-well plates, and grown for one day. Cells were then serum starved overnight and treated with the given concentrations of TGFβ-1 for a period of 24 hours. ELISA assays (R&D systems, Minneapolis, MN) for interleukin-8 and interleukin-11 cytokines were performed using 50 µL of the cell culture media.
RNA silencing and SMAD4 overexpression studies.
MDA-MB-231 (hm) cells plated at a density of 2 × 105 cells/well of a 24 well plate and treated with the given concentrations of validated stealth SMAD4 RNAis (20 or 40 nM) or MAPK14 RNAis (20 or 40 nM) and low GC content negative control RNAi (Invitrogen). Cells were transfected using Lipofectamine 2000 according to procedure outlined in the product manual. Transfection efficiency of more than 90% was determined using the BLOCK IT siRNA controls included with the respective kits. Efficiency of knockdown for each gene and the negative control was determined by western analyses. After 48 hours of incubation with the respective siRNA complexes the cells were serum starved overnight and treated with TGFβ-1 for an additional period of 24 hours. Media from these siRNA treated cells was further subjected to ELISA to measure the cytokine levels as outlined above. To examine the effect of SMAD4 overexpression on the cytokine production, MDA-MB-468 cells were transfected with pcDNA3.1 expression vector containing SMAD4 gene or a control vector (kindly provided by Dr. Asish Ghosh, Northwestern University, Chicago) using Lipofectamine 2000 for 24 hours. Incubations were continued in the absence or presence of TGF (5 ng/ml), and cell media analyzed for cytokine production.
Microscopy.
Cells cultivated on sterile cover glasses were serum starved overnight and treated with 1 ng/mL TGFβ-1 for a period of 1 hour. The cells were fixed with 3.7% formaldehyde, washed with PBS, and treated with rabbit polyclonal phosphoSMAD3 antibody (Cell Signaling) overnight at 4°C. After three washes of PBS, the cover glasses were incubated in Alexa Fluor® 488 goat antirabbit secondary antibody (Invitrogen) for one hour at room temperature. The cover glasses were washed in PBS, counterstained with DAPI and mounted using ProLong® Antifade reagent (Invitrogen).
TGFβR expression in breast cancer cells.
DNase treated total RNA was isolated from all four cell lines was reverse transcribed to cDNA using MMLV reverse transcriptase. Polymerase chain reaction using gene specific primers was further used to analyze the cDNA samples for presence of receptors TGFβRI and TGFβRII. Gene specific primer sequences are as follows: TGFβRI [tgfbr1f 5′-TGG ACT CAG CTC TGG TTG GT-3′ and tgfr1r 5′-CAC TCT GTG GTT TGG AGC AA-3′] TGFβRII [tgfβII93f 5′-GGT TAA TAA CGA CAT GAT AGT CAC TG-3′ and tgfβII403r 5′-TGA AGA AAG TCT CAC CAG GCT-3′] and β-actin [actbf 5′-CCA CGA AAC TAC CTT CAA CT-3′ and actbr 5′-CAT ACT CCT GCT TGC TGA TC-3′].
Abbreviations
| TGFβ | = | transforming growth factorβ |
| IL-11 | = | interleukin-11 |
| IL-8 | = | interleukin-8 |
| TGFβRI | = | TGFβ receptorI |
| TGFβRII | = | TGFβ receptorII |
| MAPK | = | MAP kinase |
Figures and Tables
Figure 1 The effect of TGFβ on the IL-11 and IL-8 levels in breast cancer cell lines. Various cells shown were grown, serum starved and exposed to TGFβ-1 (1 ng/ml) for 24 hours. IL-11 and IL-8 were measured by ELISA. The graph represents the quantities of cytokines IL-11 (A), IL-8 (B) secreted by various cells shown. The data is representative of more than two individual experiments with similar results.
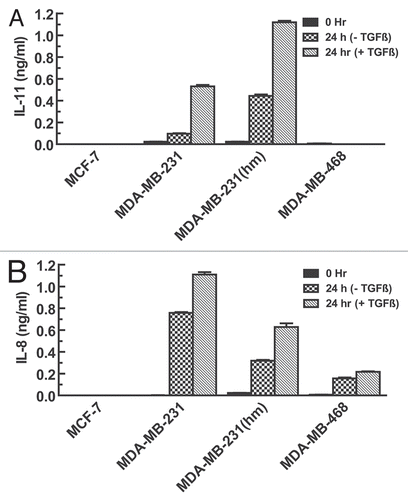
Figure 2 Effects of TGFβ-1 on phosphorylation of SMAD3 and p38 MAPK in various breast cancer cells. (A) Cells were stimulated with TGFβ1 (1 ng/ml or 5 ng/ml) for 60 minutes and subjected to western blot analysis to measure phospho SMAD3 levels using phospho SMAD3 antibody. Equal loading of the lanes was confirmed by stripping and re-probing the blots with total SMAD2/3 antibody. (B) Cells were cultured in the presence or absence of 5 ng/ml TGFβ1 for 60 minutes and p38 MAPK levels determined by probing with phospho p38 antibody. Equal protein loading was confirmed by stripping and re-probing the blots with total p38 MAPK antibody.
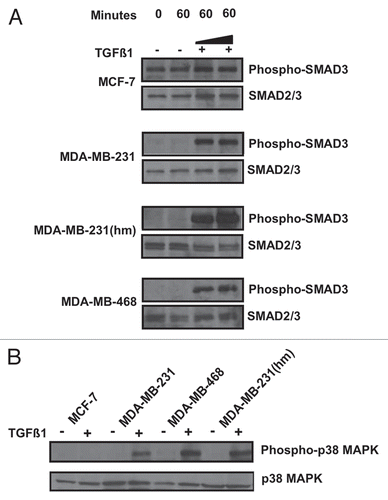
Figure 3 Effect of RNA silencing, and SMAD4 overexpression on IL11 and IL-8 levels in breast cancer cells. MDA-MB-231 (hm) cells were treated with the stealth SMAD4 RNAis (20 or 40 nM) (A) or 20 nM (B and C) or MAPK14 RNAis (40 nM) (D) and low GC content negative control RNAi, in the presence of TGFβ-1 (2 ng/ml). (A) Shows western blot analysis of SMAD4 and actin expression in the cell lysates. (B and C) Media from RNAis treated cells were used to measure the IL-11 or IL-8 levels by ELISA. (E) To examine the effect of SMAD4 overexpression on the cytokine production, MDA-MB-468 cells were transfected with pcDNA3.1 expression vector containing SMAD4 gene or a control vector, in the absence or presence of TGF (5 ng/ml), and cell media analyzed for cytokine production.
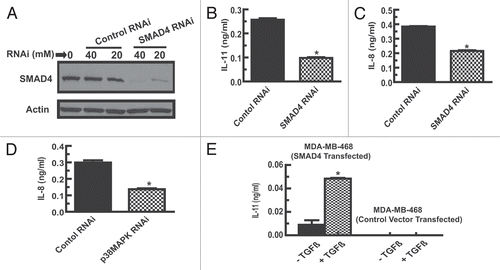
Figure 4 Analysis of TGFβRI and TGFβRII, and SMAD4 expression in breast cancer cells. (A) Total RNA from various cell lines was converted to cDNA. PCR using gene specific primers amplifying a gene segment was conducted to analyze the expression of TGFβRI and TGFβRII receptors. Human β-actin was used as a reference gene. (B) Western blot analysis of the four breast cancer cell lines for expression of SMAD4 protein. The blots were stripped and re-probed with total SMAD2/3 antibody to confirm protein loading.
Figure 5 Effect of TGFβ-1 on phospho-SMAD3 translocation to the nuclei in various breast cancer cell lines. Serum starved breast cancer cells were untreated or treated with 1 ng/ml TGFβ1 for 60 minutes before immunofluorescent staining with phospho-SMAD3 antibody. Nuclei were counterstained with DAPI.
Acknowledgements
Authors thank Dr. Janardan Khandekar for his support; Dr. Asish Ghosh for providing pcDNA3.1 plasmid containing SMAD4 gene; and Jessica Lee for technical assistance. This work was supported by National Institutes of Health grant R01CA127380.
References
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27:165 - 176
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 2007; 11:259 - 273
- Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res 2009; 19:89 - 102
- Serganova I, Moroz E, Vider J, Gogiberidze G, Moroz M, Pillarsetty N, et al. Multimodality imaging of TGFbeta signaling in breast cancer metastases. Faseb J 2009; 23:2662 - 2672
- Guise TA. Breaking down bone: new insight into site-specific mechanisms of breast cancer osteolysis mediated by metalloproteinases. Genes Dev 2009; 23:2117 - 2123
- Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol 2009; 310:71 - 81
- Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 2006; 12:6213 - 6216
- Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 2005; 102:13909 - 13914
- Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 2005; 65:11001 - 11009
- Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P. Biology of breast cancer bone metastasis. Cancer Biol Ther 2007; 7:3 - 9
- Wendt MK, Smith JA, Schiemann WP. p130Cas is required for mammary tumor growth and transforming growth factor-beta-mediated metastasis through regulation of Smad2/3 activity. J Biol Chem 2009; 284:34145 - 34156
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGFbeta family signalling. Nature 2003; 425:577 - 584
- Zhang YE. Non-Smad pathways in TGFbeta signaling. Cell Res 2009; 19:128 - 139
- Shi Y, Massague J. Mechanisms of TGFbeta signaling from cell membrane to the nucleus. Cell 2003; 113:685 - 700
- Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther 2007; 6:2609 - 2617
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003; 3:537 - 549
- Iyer I, Wang ZG, Akhtari M, Zhao W, Seth P. Targeting TGFbeta signaling for cancer therapy. Cancer Biol Ther 2005; 4:261 - 266
- Biswas S, Criswell TL, Wang SE, Arteaga CL. Inhibition of transforming growth factor-beta signaling in human cancer: targeting a tumor suppressor network as a therapeutic strategy. Clin Cancer Res 2006; 12:4142 - 4146
- Hu Z, Robbins JS, Pister A, Zafar MB, Zhang ZW, Gupta J, et al. A modified hTERT promoter-directed oncolytic adenovirus replication with concurrent inhibition of TGFbeta signaling for breast cancer therapy. Cancer Gene Ther 2010; 17:235 - 243
- Seth P, Wang ZG, Pister A, Zafar MB, Kim S, Guise T, et al. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factorbeta receptor II and human immunoglobulin Fc for breast cancer therapy. Hum Gene Ther 2006; 17:1152 - 1160
- Arteaga CL. Inhibition of TGFbeta signaling in cancer therapy. Curr Opin Genet Dev 2006; 16:30 - 37
- Katayose D, Gudas J, Nguyen H, Srivastava S, Cowan KH, Seth P. Cytotoxic effects of adenovirus-mediated wild-type p53 protein expression in normal and tumor mammary epithelial cells. Clin Cancer Res 1995; 1:889 - 897