Abstract
Bortezomib is well-known for inducing cell death in cancer cells, specifically through the mechanism of proteasome inhibition. Thiostrepton, a thiazole antibiotic, has also been described for its proteasome inhibitory action, although differing slightly to bortezomib in the proteasomal site to which it is active. Previously we had shown the synergic effect of bortezomib and thiostrepton in breast cancer cells in vitro, where sub-apoptotic concentrations of both proteasome inhibitors resulted in synergic increase in cell death when combined as a treatment. Here, we administered such a combination to MDA-MB-231 xenograft tumors in vivo, and found that the effect of complementary proteasome inhibitors reduced tumor growth rates more efficiently than compared with when administered alone. Increased induction of apoptotic activity in tumors was found be associated with the growth inhibitory activity of combination treatment. Further examination additionally revealed that combination-treated tumors exhibited reduced proteasome activity, compared with non-treated and single drug-treated tumors. These data suggest that this drug combination may be useful as a therapy for solid tumors.
Introduction
By interfering with inherent mechanisms for balancing protein levels, proteasome inhibitors have shown potent effects for inducing apoptosis in cancer cells. Proteasome inhibitors operate by direct or indirect inhibition of the enzymatic sites within the proteasome, resulting in the prevention of protein degradation which leads to the accumulation of specific proteins that affect cell viability. For example, treatment with proteasome inhibitors leads to the accumulation of phosphorylated IκB-α, a regulatory protein responsible for preventing the nuclear translocation of NF-κB.Citation1,Citation2 By stabilizing p-IκB-α, the transcriptional activity of NF-κB is prevented, halting the transcription of proliferation-associated genes.Citation3,Citation4 However, the effect of proteasome inhibitors on NF-κB cannot fully explain their anticancer activities.Citation5 Bortezomib, the first FDA-approved proteasome inhibitor for use as a chemotherapeutic drug for cancer treatment,Citation6,Citation7 is well characterized for its direct inhibition of two of three of the specific binding sites of the proteasome.Citation8 The thiazole antibiotic, thiostrepton, is a more recently discovered proteasome inhibitor which has been shown to inhibit the third site of the proteasome,Citation9 to result in cell death in a variety of cancer cell lines.Citation10,Citation11 The allosteric effect of complementary proteasome inhibitors has been discussed, where the inhibition of the “caspase-like” site, the one inhibited by thiostrepton, can sensitize inhibition of the other “chymotrypsin-like” and “trypsin-like” sites by drugs such as bortezomib.Citation12 The synergic anticancer effect of combining these two complementary proteasome inhibitors has been further described in a range of cancer cell lines in vitro.Citation13,Citation14 Other studies have also identified the use of complementary proteasome inhibitors for the treatment of myeloma cancers, leaving room for the development of such combination cocktails for other types of tumor.Citation15 Therefore, we aimed to investigate the effect of combining thiostrepton with bortezomib as an anticancer drug for treating triple negative xenograft breast cancer tumors.
Thiostrepton is a highly hydrophobic molecule, therefore methods for its solubilization into aqueous solutions are required to demonstrate its potential as a drug for cancer therapy. Previously described are the moderate anticancer effects of thiostrepton in breast cancer xenograft model, most probably due to the solubility issues of the drug.Citation10 Since, we have contemplated the solubilization of thiostrepton into micellar nanoparticles, thereby addressing the issue with solubility and at the same time formulated a thiostrepton-encapsulated nanomedicine for improved delivery to cancers. The advantages of employing nanoparticle vehicles for delivering cancer drugs lie in both the protection of the drug from external stimuli during transport (in circulation) and in the increase of the concentration of drugs in the tumor. The vasculature that supplies tumors differsfrom that of normal organs in their irregularity and their punctuations with fenestrations with diameters of approximately 700 nm.Citation16 Having considered the solubility issues of thiostrepton, we report on the study of combining the complementary proteasome inhibitors, thiostrepton and bortezomib, for the treatment of solid breast cancer tumor xenografts.
Results and Discussion
Thiostrepton solubilization was performed by its encapsulation into nanomeric particles. According to a previous protocol, nanoparticle formulation was achieved using DSPE-PEG2000MeO, an amphiphilic polymer-lipid conjugate as the material for thiostrepton encapsulation.Citation17 Upon dispersion into aqueous solution, DSPE-PEG2000-MeO self-assembles into nanoparticular structures, where the lipidic compartments point inwards to form hydrophobic cores in which other hydrophobic molecules can be solubilized. It is within this hydrophobic core that thiostrepton is entrapped, resulting in a nanoparticle micelle structure approximately 100 nm in diameter, a size small enough to pass through such fenestrations to selectively accumulate into tumors. The ability of micelle-thiostrepton to localize into solid tumors was examined by fluorescently labeling the nanoparticle and measuring the amount of tumor-associated fluorescence over time. We had found that fluorescence within the tumor was highest at 4 h post-administration, whereas by 17 h, the tumor-associated fluorescence had predominately diminished (). This was demonstrative that micelle-thiostrepton was able to reach tumors at high concentrations after intravenous injection, upon which its anticancer effects can be exerted.
To determine the combinatory effect of bortezomib and thiostrepton, animals bearing triple-negative MDA-MB-231-luc solid breast tumor xenografts were administered with either bortezomib (0.5 mg/kg), micelle-encapsulated thiostrepton (30 mg/kg) or a combination of the two. The in vitro synergic effect of this combination for inducing apoptosis in the MDAMB-231 cell line was previously described.Citation13 After a dosing schedule of five injections (15 d, 2–3 injections per week), tumors treated with a combination of the proteasome inhibitors appeared to be significantly smaller in size, compared with non-treated tumors and to those treated with single drugs (). Bortezomib and micelle-thiostrepton-treated tumors progressed in size at a rate similar to that of non-treated tumors, although a slight reduction in tumor growth was observed after repeated treatment (). After completion of the treatment schedule, non-treated tumors had increased in size (compared with day 0, first day of treatment) by 10-fold, bortezomib and thiostreptontreated tumors had increased in size by 6-fold, whereas tumors treated with a combination of the drugs only increased in volume by 3-fold (). This is depicting the enhanced effect of treating solid tumors with a combination of proteasome inhibitors. The implanted tumor cells also stably express luciferase, therefore live imaging of tumor-associated bioluminescence also allowed the determination of tumor cell viability. Bioluminescence data correlates with that of the tumor growth curve, where non-treated tumors expressed higher amounts of luciferase, compared with tumors treated with a combination of proteasome inhibitors (). After the treatment schedule, only animals treated with a combination of proteasome inhibitors showed weight loss, probably a result of both the inhibition of tumor growth (less tumor mass) and the slight increase in toxicity from combination treatment. However, it is likely that any toxicity is far from lethal, as the weight loss was a maximum of 5% ()
Upon completion of the dosing program, tumors were harvested and examined for markers of apoptosis. Immunohistochemical analysis on individual tumors demonstrated enhanced induction of cleaved caspase-3 in combination-treated tumors, compared with non-treated tumors (). Furthermore, individual homogenized tumor samples also showed increased promotion of cleaved-PARP, another apoptotic marker, in combination-treated tumors compared with non-treated tumors (). This suggests that the suppression of tumor growth by combination treatments is associated with induction of cell death within the tumor cells. Tumor homogenates were also examined for differences in proteasome activities of tumor-associated proteasomes. Homogenized tumor lysates were incubated with the fluorogenic peptide YVAD-amc, a proteasome substrate that is cleaved by the caspase-like site within the proteasome. First, it was found that tumor-associated proteasomes from non-treated tumors maintained the highest activity (). Contrastingly, tumors treated with a combination of the proteasome inhibitors bortezomib and thiostrepton exhibited impaired proteasome function, as demonstrated by the reduced abilities of tumor-associated proteasomes to cleave the fluorogenic substrate (). Furthermore, single drug-treated tumors also showed reduction in proteasome substrate cleavage, however to a lesser extent than tumors treated with the combination of complementary inhibitors. This may the reason as to why a bortezomib-only treatment proves challenging in treating solid tumors.Citation18 The decrease in proteasome activity in combination-treated tumors is likely a result of proteasome inhibition by both bortezomib and thiostrepton, which ultimately leads to the suppression of tumor growth.Citation11,Citation13 Similar effect is observed when the combination of such complementary proteasome inhibitors is used to inhibit the activity of purified proteasomes (). Bortezomib and thiostrepton alone only slightly hindered the effect of purified proteasomes to cleave YVAD-amc substrates, whereas the effect of proteasome obstruction was more apparent when used in combination (). Although we believe that anticancer effects of thiostrepton and other proteasome inhibitors may be linked to the suppression of FOXM1, the exact mechanism of this suppression is not completely understood.Citation19,Citation20
Here, the main aim of this communication is to demonstrate the complementary effect of thiostrepton plus bortezomib on inhibition of breast xenograft tumor growth in vivo. Our data suggest that the anticancer effect of combination of thiostrepton and bortezomib is superior to their individual treatments and may have a potential as a therapy against solid tumors. Further study is required to elucidate the full mechanisms through which tumor growth is inhibited by combined administration of these two proteasome inhibitors and their relevance to patient treatment.
Material and Methods
MDA-MB-231-luc-D3H2-LN, human lymph node-derived metastatic mammary gland adenocarcinoma (Caliper Lifescience) were maintained in MEM media (Mediatech) supplemented with 10% FBS (Atlanta Biological), 1% 100x non-essential amino acids (Gibco), 1% 200 mM NaPyruvate (Gibco) and 75 µg/mL Zeocin (Invitrogen). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000-MeO) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rhodamine-DOPE) was purchased from Avanti Polar Lipids. Thiostrepton (from Streptomyces azureus, 90% purity) was obtained from Sigma and bortezomib was purchased from Millennium pharmaceutics. Purified proteasomes and the YVAD-amc proteasome substrate were purchased from Enzo.
Thiostrepton-nanoparticle preparation.
A thin lipid-film of DSPE-PEG2000-MeO and thiostrepton was prepared by mixing the two components in chloroform in a 10 mL round-bottomed flask. The chloroform was removed in vacuo and by air-drying, after which the lipid film was hydrated (for final thiostrepton concentration of 1 or 2 mM) with PBS (pH7) or H2O and vortexed at room temperature for 10 min. Final solutions contained 1 mM of thiostrepton contained in 3 mM of DSPE-PEG2000-MeO. For fluorescence labeling, DOPE-Rhodamine was incorporated at the chloroform stage at a 1:100 molar ratio to DSPE-PEG2000-MeO. Sizes and zetapotentials of nanoparticles (in PBS) were analyzed by dynamic light scattering, using a 5 mW 633 nm laser angled at 90° to the sample.Citation20
Animal maintenance and tumor xenograft experiments.
Animals were maintained and treated in accordance with the Animal Care and Use Committee of UIC. Tumor models were prepared by implanting 1 x 106 of MDA-MB-231-luc cells suspended in 50 uL of 1:1 PBS/Matrigel into each flank of 4-week-old male athymic mice (Taconic). Treatment began once tumors reached sizes of 100 mm3. After completion of the dosing schedule, animals were sacrificed and tumors were removed. Tumors were frozen in liquid N2 and kept at -80°C until further analysis.
Biodistribution of micelle-thiostrepton nanoparticles.
Rho-labeled micelle-thiostrepton nanoparticles (1mM in PBS) were injected via the tail-vein into MDA-MB-231 tumor-bearing animals (1 cm3 per tumor) at a dose of 40mg thiostrepton/kg. At 4, 17 and 24 h post-injection, animals were sacrificed, and organs were removed and imaged using the Xenogen IVIS system for rhodaminefluorescence distribution (ex = 570 nm, em = 620 nm). Background autofluorescence was eliminated using a spectral unmixing algorithm provided by Living Image software and photon flux per organ was measured again with Living Image software.
Treatment of xenograft models with micelle-encapsulated thiostrepton and bortezomib.
Animals bearing tumors were randomized into groups of four to five, where groups were administered with either 30 mg/kg of thiostrepton encapsulated in micelles (2 mM thiostrepton/6 mM DSPE-PEG2000-MeO in PBS, 200–300 µL i/v), 0.5 mg/kg Bortezomib (in saline, 50 µL per injection i/p) or with both thiostrepton 30 mg/kg and bortezomib 0.5 mg/kg. Treatments were performed once every 2 d (three times a week), during which tumor volumes were monitored with calipers (l x w x h = volume, mm3). Animals were also weighed once a week. After five treatments, animals were sacrificed and tumors removed. Removed tumors were divided into two pieces, one piece was fixed in 10% formalin (for 24 h, followed by storage in 70% EtOH solution) and the other was frozen in liquid N2 and mechanically homogenized (Fisher, Polytron) in IP lysis buffer (20 mM HEPES, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 100 mM NaF, 10 mM Na4P2O7, 1 mM Na3VO4, 0.2 mM PMSF).
For bioluminescence imaging, animals were injected intraperitonially with 100 mg luciferin/kg 10 min before animals were anaesthetized under isoflurane. Animals were then imaged for tumor-associated bioluminescence (IVIS, Xenogen), where relative units of bioluminescence were quantified as flux (photon/sec) and analyzed with Living Image software.
Immunohistochemical and western blot analysis of tumor tissue.
Formalin-fixed tumor tissues were embedded in paraffin and sliced to 4-µm-thick sections. Sections were deparaffinzed by submerging sample-containing slides into a series of solvents of decreasing hydrophobicity. Heating samples in 10 mM citric acid (pH 6) was used for antigen retrieval. After blocking with 3% H2O2 in MeOH, and further blocking in normal goat serum in PBS, slides were treated with primary anti-anti-cleaved caspase-3 or control IgG antibodies at concentrations of 1:100, in 1% BSA/PBS. Biotinylated secondary antibody treatment and Avidin HPR treatment was performed according to the Vectastain ABC kit manual (anti-rabbit, Vector Labs), followed by staining with DAB (Sigma, D-0426). Samples were further counterstained with hematoxylin (Vector Labs) before dehydration in a series of solvents and mounted with Permount (Fisher) mounting agent. Slides were analyzed on a Zeiss Apotome microscope. For western blot analysis, protein concentrations of tumor homogenates were measured by the Bio-Rad Protein Assay, after which protein separation was performed on 8 or 12% SDS-PAGE gels. Separated proteins were then transferred onto PVDF membranes (Millipore) and immunoblotted with specific antibodies against cleaved PARP (h-250, Santa Cruz) and β-actin (Sigma).
Proteasome activity assay.
Homogenized tumor lysates were measured for protein concentration with the Bio-Rad Protein assay to determine appropriate lysate volumes corresponding to 40 µg of protein. Consequently, to each reaction vial was added tumor lysates (40 µg, ∼10 µL), 10 x assay buffer (10 µL, 250 mM HEPES, pH 7.5, 5 mM EDTA, 0.5% NP-40, 0.01% SDS w/v), Ac-YVAD-amc substrate (10 µL, 0.5 mM) and H2O (∼70 µL). Assay mixtures were incubated at 60°C for 2 h, after which solutions were examined for fluorescence at ex = 340 nm and em = 505 nm. Values are represented as a percent of the proteasome activity of non-treated tumors.
To assay proteasome inhibitor activities of proteasome inhibitors purified proteasomes, (0.5 µg, 1 µg/µL) were pre-incubated with inhibitors bortezomib (5 nM, 1 µL of 0.5 µM in DMSO) and/or thiostrepton (20 µM, 1 µL of 2 µM) in 10x assay buffer (10 µL) and H2O (88.5 µL) for 30 min at room temperature. Ac-YVAD-amc substrate (10 µL, 0.5 mM) was then added and the assay solution was incubated at 60°C for 2 h, after which solutions were examined for fluorescence at ex = 340 nm and em = 505 nm. Values are represented as a percent of the proteasome activity of non-inhibited proteasomes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 Effect of bortezomib and micelle-thiostrepton treatment on MDA-MB-231-luc tumor xenograft models. (A) Biodistribution of rhodamine-labeled micelle-thiostrepton complexes in tumor-bearing mice. High micelle-associated fluorescence is apparent in tumors and in the liver 4 h post-injection. Value for each organ represents an average of n = 2–4. (B) Anticancer effects of bortezomib (0.5 mg/kg) alone, micelle-thiostrepton treatment (30 mg/kg) alone, a combination of bortezomib and micelle-thiostrepton on MDA-MB-231 xenograft volumes, after five treatments over 15 d, n = 6–8. All averages were considered significant, and the t-test p value of the non-treated and the V+T-treated tumors was calculated to be 0.011. Error bars represent SEM. (C) Change in tumor-associated bioluminescence in animals bearing breast cancer xenografts after continuous treatment with a combination of complementary proteasome inhibitors over 15 d. Luciferase expression demonstrates that the sizes of tumors treated with bortezomib and thiostrepton were much smaller in size than the non-treated controls. (D) Change in animal weight during treatment schedule. Only the animals receiving the combination of proteasome inhibitors demonstrated slight weight loss, compared with non-treated and single drug-treated animals.
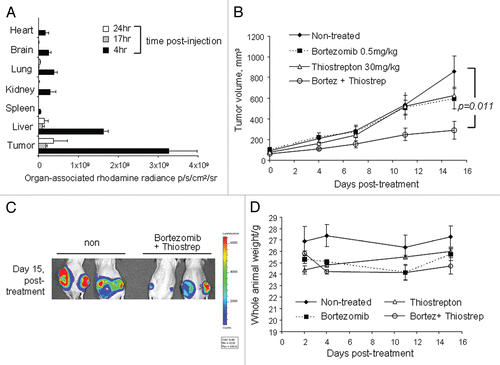
Figure 2 Combination treatment with bortezomib and micelle-thiostrepton induces cell death in MDA-MB-231-luc tumor xenografts, as determined by promotion of apoptotic markers. (A) Immunhistochemical analysis of four individual tumors (two from non-treated and two from combination treated groups) for cleaved caspase-3 expression (counterstained with hematoxylin). Cleaved-caspase-3 is evidently enhanced in combination-treated tumors, identified by the presence of larger numbers of dark, DAB-stained clusters. Control slides were incubated with rabbit IgG primary antibodies. Blue bars represent 100 µm (20 x). (B) Protein gel blotting of a panel of individual tumor homogenates (five of non-treated and five of combination-treated) against cleaved PARP and β-actin. Cleaved PARP expression is increased in tumors treated with a combination of thiostrepton and bortezomib.
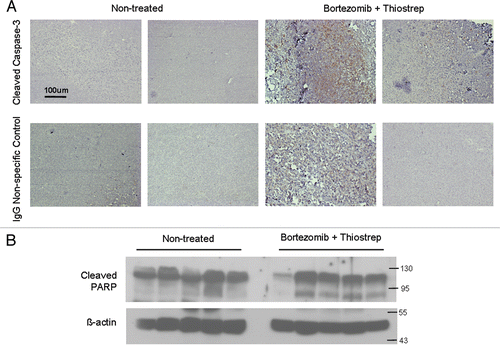
Figure 3 Combination of bortezomib and thiostrepton suppresses proteasome activity. (A) Proteasome activity assay showed that lysates from MDA-MB-231 tumors treated with a combination of proteasome inhibitors had decreased ability for proteasome substrate cleavage, compared with that from non-treated and single drug-treated tumors. Values represent averages of 6–8 tumors and error bars depict SEM p values are stated within the figure. (B) Proteasome activity assay showed that bortezomib and thiostrepton inhibit proteasome activities of purified proteasomes to a greater extent when used in combination.
Acknowledgments
We thank Dr. Angela Tyner (UIC) for the gift of the MDAMB-231-luc cells. This study was funded by NIH grants 1RO1CA129414 and 1R21CA134615 (to A.L.G.).
References
- Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J 1994; 13:5433 - 5441; PMID: 7957109
- Wang CY, Mayo MW, Baldwin AS. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 1996; 274:784 - 787; PMID: 8864119; http://dx.doi.org/10.1126/science.274.5288.784
- Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J 1995; 14:2876 - 2883; PMID: 7796813
- Orlowski RZ, Baldwin AS. NF-kappaB as a therapeutic target in cancer. Trends Mol Med 2002; 8:385 - 389; PMID: 12127724; http://dx.doi.org/10.1016/S14714914(02)02375-4
- Kandel ES. NFkappaB inhibition and more: a side-by-side comparison of the inhibitors of IKK and proteasome. Cell Cycle 2009; 8:1819 - 1820; PMID: 19471125; http://dx.doi.org/10.4161/cc.8.12.8966
- Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 2002; 8:2505 - 2511; PMID: 12171876
- Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 2004; 22:2108 - 2121; PMID: 15169797; http://dx.doi.org/10.1200/JCO.2004.02.106
- Crawford LJ, Walker B, Ovaa H, Chauhan D, Anderson KC, Morris TC, et al. Comparative selectivity and specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res 2006; 66:6379 - 6386; PMID: 16778216; http://dx.doi.org/10.1158/0008-5472.CAN-06-0605
- Schoof S, Pradel G, Aminake M, Ellinger B, Baumann S, Potowski M, et al. Antiplasmodial thiostrepton derivatives: proteasome inhibitors with a dual mode of action. Angew Chem Int Ed Engl 2010; 49:3317 - 3321; PMID: 20358566
- Halasi M, Zhao H, Dahari H, Bhat U, Gonzalez E, Lyubimov A, et al. Thiazole antibiotics against breast cancer. Cell Cycle 2010; 9:1214 - 1217; PMID: 20410687; http://dx.doi.org/10.4161/cc.9.6.10955
- Bhat UG, Halasi M, Gartel A. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS ONE 2009; 4:e5592; PMID: 19440351; http://dx.doi.org/10.1371/journal.pone.0005592
- Britton M, Lucas MM, Downey SL, Screen M, Pletnev AA, Verdoes M, et al. Selective inhibitor of proteasome's caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites. Chem Biol 2009; 16:1278 - 1289; PMID: 20064438; http://dx.doi.org/10.1016/j.chembiol.2009.11.015
- Pandit B, Gartel AL. Thiazole antibiotic thiostrepton synergize with bortezomib to induce apoptosis in cancer cells. PLoS ONE 2011; 6:e17110; PMID: 21365012; http://dx.doi.org/10.1371/journal.pone.0017110
- Pandit B, Gartel AL. New potential anti-cancer agents synergize with bortezomib and ABT-737 against prostate cancer. Prostate 2010; 70:825 - 833; PMID: 20058240
- Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood 2008; 111:1654 - 1664; PMID: 18006697; http://dx.doi.org/10.1182/blood-2007-08-105601
- Roberts WG, Delaat J, Nagane M, Huang S, Cavenee WK, Palade GE. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol 1998; 153:1239 - 1248; PMID: 9777955; http://dx.doi.org/10.1016/S0002-9440(10)65668-4
- Wang M, Gartel AL. Micelle-encapsulated thiostrepton as an effective nanomedicine for inhibiting tumor growth and for suppressing FOXM1 in human xenografts. Mol Cancer Ther 2011; In press. PMID: 21903609; PMID: 10.1158/1535-7163.MCT-11-0536
- Caravita T, de Fabritiis P, Palumbo A, Amadori S, Boccadoro M. Bortezomib: efficacy comparisons in solid tumors and hematologic malignancies. Nat Clin Pract Oncol 2006; 3:374 - 387; PMID: 16826218; http://dx.doi.org/10.1038/ncponc0555
- Hegde NS, Sanders DA, Rodriguez R, Balasubramanian S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem 2011; 3:725 - 731; PMID: 21860463; http://dx.doi.org/10.1038/nchem.1114
- Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, et al. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm Res 2003; 20:584 - 590; PMID: 12739765; http://dx.doi.org/10.1023/A:1023290514575