Abstract
Objective
Thymosin β-4 (Tβ4) is a ubiquitous peptide that plays pivotal roles in the cytoskeletal system and in cell differentiation during embryogenesis. Recently, a role for Tβ4 has been proposed in experimental and human carcinogenesis. This study was aimed at evaluating the correlation between Tβ4 immunoractivity and colorectal cancer, with particular attemption to tumor cells undergoing epithelial-mesenchymal transition.
Methods and Results
86 intestinal biopsies were retrospectively analyzed including 76 colorectal adenocarcinomas with evident features of epithelial-mesenchymal transition, and 10 samples of normal colorectal mucosa. Paraffin sections were immunostained for Tβ4 and for E-cadherin. Total RNA was isolated from frozen specimens obtained, at surgery, from the normal colon mucosa, the deeper regions and the superficial tumor regions in four cases of colon cancer. Tβ4 immunoreactivity was detected in the vast majority (59/76) of colon carcinomas, showing a patchy distribution, with well differentiated areas significantly more reactive than the less differentiated tumor zones. We also noted a zonal pattern in the majority of tumors, characterized by a progressive increase in immunostaining for Tβ4 from the superficial toward the deepest tumor regions. The strongest expression for Tβ4 was frequently detected in invading tumor cells with features of epithelial-mesenchymal transition. The increase in reactivity for Tβ4 matched with a progressive decrease in E-cadherin expression in invading cancer cells. At mRNA level, the differences in Tβ4 expression between the surrounding colon mucosa and the tumors samples were not significant.
Conclusions
Our data show that Tβ4 is expressed in the majority of colon cancers, with preferential immunoreactivity in deep tumor regions. The preferential expression of the peptide and the increase in intensity of the immunostaining at the invasion front suggests a possible link between the peptide and the process of epithelial mesenchymal transition, suggesting a role for Tβ4 in colorectal cancer invasion and metastasis.
Introduction
Epithelial-mesenchymal transition (EMT) is a complex process characterized by the loss of original epithelial features in embryonic and in tumor cells, associated with the gain of a mesenchymal phenotype and producing non-polarized isolated cells embedded in the extracellular matrix.Citation1 At molecular level, EMT requires multiple events, such as disruption of intercellular junctions, loss of cell polarity, microtubule disruption and basement membrane breakdown.Citation2 EMT has been originally described by embriologists as a key process in many developmental processes,Citation3 including the formation of the neural crest and of the myotome.Citation4 Recently, EMT has emerged to be a key step in cancer progression, allowing tumor cells to acquire an invasive behavior and disseminate.Citation5,Citation6 EMT is now believed to be a major mechanism by which cancer cells become invasive, able to translocate from the initial neoplastic core, to penetrate vessel endothelium, entering circulation thus forming distant metastases.Citation7,Citation8
The typical hystologic expression of EMT may be observed at the infiltrative margins of carcinomas, as individual malignant cells, often acquiring a spindle shape, detach from the tumor mass, and stay independently within the interstitial matrix of the peritumoral stroma.Citation8 At immunohistochemistry, EMT is characterized by a dramatic decrease in intercellular expression of E-cadherin,Citation9 which was postulated to be the result of adherens junctions disruption.Citation10
In colorectal cancer, EMT is evidenced by a change in tumor tissue architecture at the deep invasive tumor margins. This particular modification of cancer architecture has been referred as “budding margins,” namely infiltrative margins with solid cell nests formed by 2–3 cancer cells, which display their acquisition of motility by infiltrating the peritumoral connective tissue.Citation11
Molecular studies have revealed that EMT consists of a number of cellular events, among which controlled basal membrane breakdown plays a crucial role. A new model for EMT mechanism has been proposed and new sequence in the distinct cellular steps which eventually lead to EMT has been evidenced.Citation12 In this model, microtubules, already known to have a central role in cell polarity and migration,Citation13 lose their stability, microtubule disruption causes basement membrane disassembly, disruption of the epithelial cell-basal membrane interaction and, eventually, breakdown of the basal membrane.Citation14 Interestingly, immunoreactivity for the anti-βtubulin antibody 6GT, which recognizes a sub-population of microtubules restricted at the basal regions of epithelial cells, progressively decreases in cells undergoing EMT, evidencing the destabilization of microtubules, followed by basal membrane disassembly and loss of epithelial characteristics by tumor cells.
Recently, Tβ4 a member of a highly conserved family of 40–44 amino acid peptides that regulate actin polimerization by binding and sequestering monomeric G-actin,Citation15 has been hypotesized to trigger EMT in colorectal carcinoma by upregulating integrin-linked kinase (ILK).Citation16 Overexpression of Tβ4 has been shown to upregulate ILK,Citation17 and consequently to cause the suppression of E-cadherin expression, resulting in disruption of adherens junctions and induction of EMT.Citation9
In light of the foregoing data, focus of this study was to assess the pattern of immunoreactivity of Tβ4 in colorectal cancer, and, in particular, the expression of this peptide in deep infiltrative margins in association with epithelial mesenchymal transition of cancer cells.
Results
Three main patterns of immunostaining for Tβ4 were observed: a punctuated and granular cytoplasmic staining, localized in the entire cytoplasm or in basal or apical regions of enterocytes; a spot-like staining, localized in the perinuclear regions and representing, possibly, a localization of the peptide at the Trans-Golgi network; a homogeneous staining diffusely distributed in the entire cytoplasm. Tβ4 expression was always restricted to the cytoplasm of normal, dysplastic and tumor cells. No nuclear reactivity was detected.
Normal colonic mucosa
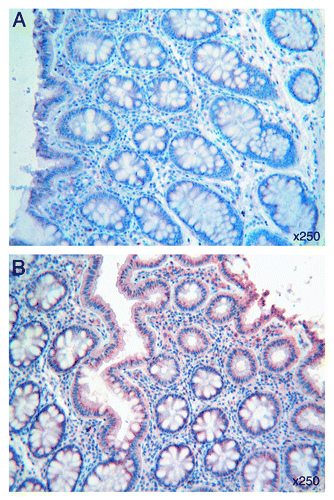
All specimens of human colonic mucosa expressed Tβ4: immunostaining for the peptide was observed in the superficial epithelium as well as in crypt epithelial cells (). The intensity of reactivity for Tβ4 was variable from a case to the next, ranging from a diffuse to a focal pattern. Immunostaining was observed in the cytoplasm of surface colon epithelium: it appeared homogeneous or punctate, and was mainly localized at the base of the cell or at apical cell regions, along the brush border. These different types of immunoreactivity for Tβ4 were often found isolated. Occasionally, they were detected in the same cells (). Tβ4 was also found in granular deposits along the enterocyte surface or inside the intestinal lumen, spread over mucous secretion.
Figure 1. (A) A homogeneous and punctate immunostaining for Tβ4 is detected in the cytoplasm at the base and at apical cell regions of normal colon epithelium. Immunoreactive granular deposits are observed on the enterocyte surface and inside the intestinal lumen. (B) Tβ4 immunoreactive perinuclear spots are observed in the enterocytes of normal colonic mucosa adjacent to adenocarcinoma margins. OMx250.
Colonic mucosa surrounding adenocarcinoma
Substantial differences were observed in Tβ4 immunoreactivity when samples of normal colonic mucosa were taken adjacent to adenocarcinoma margins: perinuclear spots, suggestive for a Golgi network localization and absent in colonic mucosa distant from the tumor, appeared instead prominent in the majority of enterocytes in proximity of tumor cells ().
Adenocarcinoma
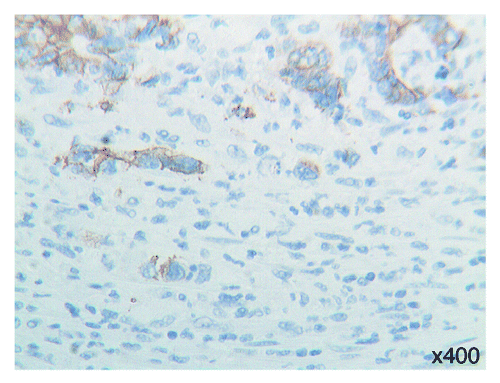
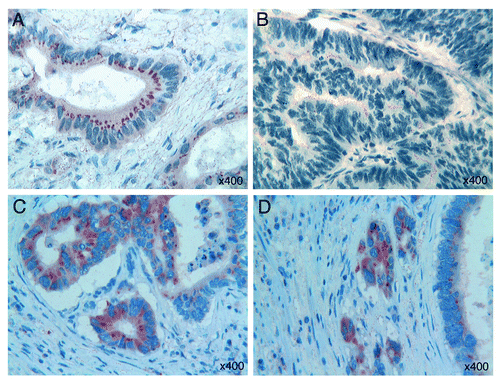
Tβ4 expression was detected in 32 out of the 46 colorectal adenocarcinomas, with differences both in the type of reactivity and in intensity. Two main patterns of immunostaining were found in colorectal adenocarcinoma: a mild fine granular reactivity and a spot-like perinuclear staining, which appeared particularly evident in tumor cells with glandular arrangement (). Striking differences in Tβ4 expression were also found inside the same tumor: in the parallel investigation of well differentiated and less differentiated or undifferentiated areas, we observed an heterogeneous distribution of the peptide, characterized by a decrease in intensity proceeding from Grade 1 to Grade 3 zones, with strongly immunoreactive areas adjacent to negative tumor zones. In less differentiated tumor zones, we frequently observed an intraluminal immunoreactivity for Tβ4 (). Immunostaining for Tβ4 was constantly restricted to the cytoplasm of tumor cells; no nuclear expression of the peptide was found. Moreover, in all 32 immunoreactive cases, we observed a positive trend in Tβ4 reactivity from superficial areas toward deeper tumor regions, at the invasive front. The highest degree of immunoreactivity for Tβ4 was always found in deepest areas of adenocarcinomas, at the invasive front with budding margins. The highest levels of reactivity for Tβ4 were detected in the cytoplasm of isolated infiltrating tumor cells undergoing EMT ().
Figure 2. (A) A fine granular Tβ4 reactivity and a spot-like perinuclear Tβ4 immunostaining are observed in colorectal tumor cells with glandular arrangement. (B) Fine granular intraluminal immunoreactivity for Tβ4 is detected in less differentiated tumor areas. (C) Strong immunoreactivity for Tβ4 in the “budding margins” of colon cancer. (D) Strong cytoplasmic immunoreactivity for Tβ4 in isolated infiltrating tumor cells with features of epithelial-mesenchymal transition. OMx400.
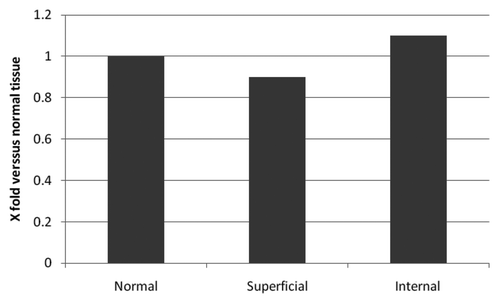
To further elucidate the significance of Tβ4 expression in the process of EMT in colon cancer cells, the status of cell-to-cell adhesion was evaluated by immunostaining for E-cadherin. As expected, a diffuse and strong membranous stainins was noted in normal mucosa. Immunostaining for E-cadherin was maintained in the central areas of adenocarcinoma. Significantly, E-cadherin immunoreactivity showed a decrease in intensity with fragmentation of the membranous staining in tumor cells undergoing EMT (). As for mRNA expression detected by RT-PCR we didn’t find any significant difference between normal colon mucosa and the deeper and superficial regions of colon cancer ().
Discussion
Thymosin β-4, a peptide named after its first detection in the calf thymus,Citation19 has been traditionally correlated with a relevant role in regulation of actin polymerization in living cells.Citation20 Tβ4 has many other biological functions: it contributes to angiogenesis,Citation21 cutaneous wound healing,Citation22 regulation of the inflammatory responseCitation23 and promotion of cell migration.Citation17 Tβ4 may also stimulate the AKT pathway, resulting in a strong anti-apoptotic effect on human cellsCitation24 as well as in developing chick motoneurons.Citation25 The antiapoptotic activity of Tβ4 has been related to its ability to inactivate caspase-3, increasing cell survival rate.Citation26 More recently, Tβ4 activity has been implicated in experimental and in human carcinogenesis.Citation27,Citation28 This hypothesis was based on the observation of Tβ4 effects on the cytoskeleton structure of cancer cells, on tumor cell motility, and on intra- and peritumoral angiogenesis. Tβ4 has been recently detected in breast cancer, in few cases of colorectal cancersCitation29 and in urotelial carcinoma.Citation9 In some cases, Tβ4 expression correlated with increased metastatic potential, thus providing a clue for the possible pro-metastatic role of the peptide.Citation30
In this study, we clearly show that Tβ4 is strongly expressed at the infiltrative front of colon cancer, particularly in the deep infiltrative margins, in tumor cells undergoing EMT (). This leads to the hypothesis that Tβ4 might be involved in cancer progression, somehow favoring cancer invasion. In previous in vitro studies, transfection of Tβ4 into a mouse melanoma cell line was shown to enhance metastatic potential of these cells, by the increase of intratumoral angiogenesis.Citation31 In a study on Tβ4 expression in human breast cancer, the peptide production was found to be upregulated inside the tumor, and mainly focused on the intratumoral vascular component, reinforcing the hypothesis that Tβ4 expression in tumor cells could be linked to angiogenesis.Citation29 In this study, we did not observe a significant immunoreactivity for Tβ4 in intratumoral and in peritumoral vessels: Tβ4 was always expressed in the cytoplasm of tumor cells, in the absence of any reactivity in the connective tissue, as previously reported by our group in developing human organs.Citation32,Citation33 Outside the tumor cells, Tβ4 immunostaining was restricted to peritumoral and intratumoral mast cells, a finding already reported in salivary gland tumorsCitation34 and in human skin.Citation35
Figure 5. Sequential steps in epithelial-mesenchymal transition at the infiltrative margin of colon cancer. Some tumor cells detached from nearest cells acquire a spindle shape and infiltrate the surrounding tissues.
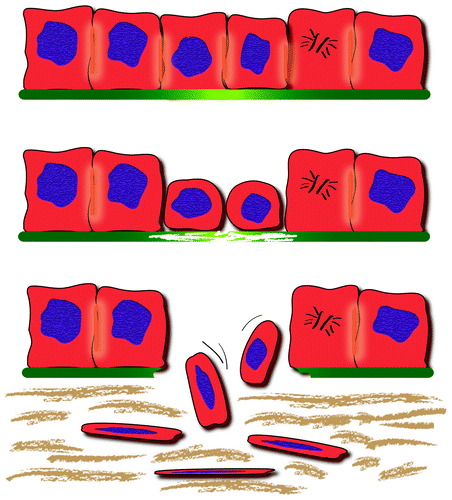
Another intriguing finding in our study is the observation of a progressive decrease in the expression of E-cadherin on the cell surface in Tβ4-reactive tumor cells undergoing EMT, with disappearance of E-cadherin in isolated spindle tumor cells migrating toward lymphatic and/or blood vessels. Since tumor cell dispersion relies on the loss of cell-cell adhesion, which is largely mediated by E-cadherin,Citation36 the strict association between Tβ4 overexpression and E-cadherin decrease in invading colon cancer cells, identifies Tβ4 as a powerful marker of EMT . On the basis of our data, we hypothesize that Tβ4 may play a critical role in promoting EMT, leading to a deregulated cell-cell adhesion through E-cadherin downregulation, as previously reported by others in urothelial carcinoma.Citation9 The absence of significant differences at mRNA level, between the normal colon mucosa and tumor tissues, may suggest a disregulation of Tβ4 expression at post-transcriptional level.
A possible role for Tβ4 in regulating motility and metastasis in non-small cell lung cancer,Citation37 in mouse fibrosarcoma,Citation38 in cultured colon cancer cellsCitation10,Citation16 and in human colon cancer cellsCitation39 has been assumed in previous studies. Our observations of a preferential localization of Tβ4 in infiltrating solid nests at the base of colorectal adenocarcinoma supports the possibility that Tβ4 expression could modulate the invading activity of colorectal cancer cells with a similar role of the one played by βIII-tubulin at the invasive margins of colon cancer.Citation40 Our hypothesis confirms previous data in colon cancer cells coltures on a role of Tβ4 as a trigger of a EMT in colo-rectal carcinoma.Citation41
Given the recent reports on the ability of Tβ4 to induce an embryonic reprogramming in adult cardiac progenitor cells, resulting in mobilization and differentiation to give rise to de novo cardiomyocytes,Citation42 we could speculate that Tβ4 re-expression in tumor cells undergoing EMT might be the sign of an embryonic reprogramming in colon cancer cells, resulting in their mobilization and migration to give rise to distant metastases. According with this hypothesis, Tβ4 could be considered as a possible target for future anticancer therapies.
In summary, in the present study we have demonstrated that Tβ4 is frequently expressed in human colorectal adenocarcinoma, with a marked preferential localization in tumor cells undergoing EMT, at the invasive front of the tumor. This evidence confirms previous hypotheses for a role of Tβ4 in facilitating the progression of colon cancer.Citation28 Identifying the molecular mechanism underlying the intimate role of Tβ4 in the process of EMT is a major challenge for future research with the prospective that inhibitors of this peptide might have a chance to become new therapeutic agents against colon cancer.
Patients and Methods
The study included archived paraffin-embedded colorectal sections obtained from 86 patients who underwent colonoscopy with biopsy or surgical colon resection. The cohort included 10 samples of normal colon mucosa and 76 colorectal adenocarcinomas. Colon cancers were included when characterized by budding margins with evident morphological signs of epithelial-mesenchymal transition.
Immunoistochemistry
For each tumor included in this study, two samples were immunostained for Tβ4. Paraffin sections were immunostained with anti-Tβ4 antibodies (Bachem-Peninsula Lab) and with anti-E-cadherin antibodies (Dako, A/S), using the labeled streptavidin-biotin complex system (LSAB2, Dako) in a Dako Autostainer (DakoCytomation). Briefly, slides were deparaffinized, rehydrated, and endogenous peroxidase activity was quenched (30 min) by 0.3% hydrogen peroxide in methanol. Slides were then subjected to heat-induced antigen retrieval by steaming unstained sections in Target Retrieval Solution (Dako TRS pH 6.1) for 30 min. Slides were incubated with 10% normal goat serum in phosphate-buffered saline (PBS) for 60 min to block non-specific binding, followed by incubation (60 min at room temperature) with the monoclonal anti-Thymosin β-4 and anti E-cadherin antibodies, diluted 1:100 in blocking solution. Slides were extensively washed with PBS containing 0.01% Triton X-100 and incubated with a secondary reagent (En Vision kit) according with the manufacturer’s (Dako) instructions. Diaminobenzidine (DAB) was used as chromogen. After additional washes, color was developed using the AEC reagent (Dako); sections were counterstained with Mayer’s hematoxylin and mounted. Sections of reactive lymph nodes with Tβ4-immunoreactive histiocytes were utilized as positive control for the immunohistochemical reaction. As negative control, the same procedure was applied omitting the primary antibody. All cases were independently reanalyzed by two pathologists specialized in gastrointestinal pathology (SN, GF), according to the 1999 WHO classification. The study protocol was approved by the Institutional Review Board.
Real Time RT-PCR
Tissue samples, obtained after surgery, from the surrounding colon mucosa, from the superficial and from the deeper tumor margins, were immediately frozen in dry ice, and kept at -70°C until lysis for RNA extraction. Total RNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions.
As internal control was used the human β actin gene. The following primers (β-act F. = 5′-GCATGGGTCAGAAGG-3′, β act R. = 5′-AGGCGTACAGGGATAG-3′, tb4 F. = 5′-GGCCACTGCGCAGACCAGACT-3′ tb4R. = 5′-CTTGATCCAACCTCTTTGCATCTTACAA-3′) were designed using the sequences of the Tymosin β-4 min RNA (GenBank accession no. NM_001101) and the human β actin mRNA (GenBank accession no. NM_001101).
Real-time reverse-transcriptase PCR analysis was perfomed in a Light Cycler apparatus (Roche) with a LightCycler-RNA Amplification kit SYBR Green I (Roche Diagnostics) according to the manufacturer’s instructions. The 20 μl final volume contained: 3 mM MgCl2, 0.25 μM of each primer 2 μl of RNA extract. Cycling was performed using the following amplification conditions: an initial reverse transcription at 55°C for 10 min, denaturation at 95°C for 30 sec followed by 35 cycles at 95°C for 10 sec, 53°C for 10 sec and 72°C for 8 sec with subsequent melting analysis: heating to 95°C for 20 sec, cooling to 45°C for 10 sec and reheating to 95°C at a rate of 0.2°C per second. Fluorescence was detected at the end of the 81°C segment in PCR step (single mode) and at 45°C segment in the melting step (continuous mode) in the F1 channel. The relative gene expression was analyzed by using the 2-ΔΔCT method.Citation18 For each analysis, three distinct biological replicas were done, and quantitative data were expressed as mean. Values of fold change in tb4 gene expression relative to the β actin RNA to above 2 or below 0.5 were considered significant.
Aknowledgments
This project was supported by Fondazione Banco di Sardegna. The authors want to thank Mr. Ignazio Ferru for secretarial assistance. The technical work of Mrs. Sandra Serra and Simonetta Paderi is also acknowledged.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Levayer R, Lecuit T. Breaking down EMT. Nat Cell Biol 2008; 10:757 - 9; http://dx.doi.org/10.1038/ncb0708-757; PMID: 18591967
- Hay ED. An overview of epithelial-mesenchymal transformation. Acta Anat (Basel) 1995; 154:8 - 20; http://dx.doi.org/10.1159/000147748; PMID: 8714286
- Thiery JP. Epithelial-mesenchymal transition in development and pathologies. Curr Opin Cell Biol 2003; 15:740 - 6; http://dx.doi.org/10.1016/j.ceb.2003.10.006; PMID: 14644200
- Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C. beta-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development 2005; 132:3895 - 905; http://dx.doi.org/10.1242/dev.01961; PMID: 16100089
- Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst 2008; 100:232 - 3; http://dx.doi.org/10.1093/jnci/djn032; PMID: 18270330
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 2008; 19:294 - 308; http://dx.doi.org/10.1016/j.semcdb.2008.02.001; PMID: 18343170
- Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 2007; 101:816 - 29; http://dx.doi.org/10.1002/jcb.21215; PMID: 17243120
- Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007; 39:305 - 18; http://dx.doi.org/10.1080/00313020701329914; PMID: 17558857
- Wang ZY, Zeng FQ, Zhu ZH, Jiang GS, Lv L, Wan F, et al. Evaluation of thymosin β4 in the regulation of epithelial-mesenchymal transformation in urothelial carcinoma. Urologic Oncology 2010; In press PMID: 20864366
- Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene 2003; 22:3297 - 306; http://dx.doi.org/10.1038/sj.onc.1206404; PMID: 12761500
- Ogawa T, Yoshida T, Tsuruta T, Tokuyama W, Adachi S, Kikuchi M, et al. Tumor budding is predictive of lymphatic involvement and lymph node metastases in submucosal invasive colorectal adenocarcinomas and in non polypoid compared with polypoid growth. Scand J Gastroenterol 2009; 4:605 - 14; http://dx.doi.org/10.1080/00365520902718911
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 2008; 10:765 - 75; http://dx.doi.org/10.1038/ncb1739; PMID: 18552836
- Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev 2007; 21:483 - 96; http://dx.doi.org/10.1101/gad.1511207; PMID: 17344411
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 2008; 10:757 - 9; http://dx.doi.org/10.1038/ncb1739; PMID: 18591967
- Goldstein AL, Hannappel E, Kleinman HK. Thymosin β4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med 2005; 11:421 - 9; http://dx.doi.org/10.1016/j.molmed.2005.07.004; PMID: 16099219
- Huang HC, Hu CH, Tang MC, Wang WS, Chen PM, Su Y. Thymosin β4 triggers an epithelial mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 2007; 26:2781 - 90; http://dx.doi.org/10.1038/sj.onc.1210078; PMID: 17072345
- Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004; 432:451 - 3; http://dx.doi.org/10.1038/nature03000; PMID: 15565135
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
- Klein JJ, Goldstein AL, White A. Enhancement of in vivo incorporation of labeled precursors into Dann and total protein of mouse lymph nodes after administration of thymic extracts. Proc Natl Acad Sci USA 1965; 53:812 - 7; http://dx.doi.org/10.1073/pnas.53.4.812; PMID: 14324539
- Sanders MC, Goldstein AL, Wang YL. Thymosin beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proc Natl Acad Sci USA 1992; 89:4678 - 82; http://dx.doi.org/10.1073/pnas.89.10.4678; PMID: 1584803
- Koutrafouri V, Leondiadis L, Avgoustakis K, Livaniou E, Czarnecki J, Ithakissios DS, et al. Effect of thymosin peptides on the chick chorioallantoic membrane angiogenesis model. Biochim Biophys Acta 2001; 1568:60 - 6; http://dx.doi.org/10.1016/S0304-4165(01)00200-8; PMID: 11731086
- Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, et al. Thymosin beta4 accelerates wound healing. J Invest Dermatol 1999; 113:364 - 8; http://dx.doi.org/10.1046/j.1523-1747.1999.00708.x; PMID: 10469335
- Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol 2003; 3:1225 - 33; http://dx.doi.org/10.1016/S1567-5769(03)00024-9; PMID: 12860178
- Tapp H, Deepe R, Ingram JA, Yarmola EG, Bubb MR, Hanley EN Jr., et al. Exogenous thymosin beta 4 prevents apoptosis in human intervertrebal annulus cells in vitro. Biotech Histochem 2009; 84:287 - 94; http://dx.doi.org/10.3109/10520290903116884; PMID: 20055734
- Choi SY, Kim DK, Eun B, Kim K, Sun W, Kim H. Anti-apoptotic function of thymosin-beta in developing chick spinal motoneurons. Biochem Biophys Res Commun 2006; 346:872 - 8; http://dx.doi.org/10.1016/j.bbrc.2006.05.207; PMID: 16782066
- Moon EY, Song JH, Yang KH. Actin-sequestering protein, thymosin-beta-4 (TB4), inhibits caspase-3 activation in paclitaxel-induced tumor cell death. Oncol Res 2007; 16:507 - 16; http://dx.doi.org/10.3727/096504007783438349; PMID: 18306930
- Larsson LI, Holck S. Localization of thymosin beta-4 in tumors. Ann N Y Acad Sci 2007; 1112:317 - 25; http://dx.doi.org/10.1196/annals.1415.005; PMID: 17495241
- Freeman K, Banyard J. B-thymosins in cancer: implications for the clinic. Future Oncol 2009; 5:755 - 8; http://dx.doi.org/10.2217/fon.09.71; PMID: 19663724
- Larsson LI, Holck S. Occurrence of thymosin beta4 in human breast cancer cells and in other cell types of the tumor microenvironment. Hum Pathol 2007; 38:114 - 9; http://dx.doi.org/10.1016/j.humpath.2006.06.025; PMID: 16949646
- Yamamoto T, Gotoh M, Kitajima M, Hirohashi S. Thymosin beta-4 expression is correlated with metastatic capacity of colorectal carcinomas. Biochem Biophys Res Commun 1993; 193:706 - 10; http://dx.doi.org/10.1006/bbrc.1993.1682; PMID: 8512568
- Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin beta4 in tumor metastasis and angiogenesis. J Natl Cancer Inst 2003; 95:1674 - 80; http://dx.doi.org/10.1093/jnci/djg100; PMID: 14625258
- Nemolato S, Messana I, Cabras T, Manconi B, Inzitari R, Fanali C, et al. Thymosin beta(4) and beta(10) levels in pre-term newborn oral cavity and foetal salivary glands evidence a switch of secretion during foetal development. PLoS ONE 2009; 4:e5109; http://dx.doi.org/10.1371/journal.pone.0005109; PMID: 19337364
- Nemolato S, Cabras T, Cau F, Fanari MU, Fanni D, Manconi B, et al. Different thymosin Beta 4 immunoreactivity in foetal and adult gastrointestinal tract. PLoS ONE 2010; 5:e9111; http://dx.doi.org/10.1371/journal.pone.0009111; PMID: 20161756
- Lai G, Nemolato S, Lecca S, Parodo G, Medda C, Faa G. The role of immunohistochemistry in the diagnosis of hyalinizing clear cell carcinoma of the minor salivary gland: a case report. Eur J Histochem 2008; 52:251 - 4; PMID: 19109100
- Nemolato S, Cabras T, Fanari MU, Cau F, Fraschini M, Manconi B, et al. Thymosin beta 4 expression in normal skin, colon mucosa and in tumor infiltrating mast cells. Eur J Histochem 2010; 54:e3; http://dx.doi.org/10.4081/ejh.2010.e3; PMID: 20353910
- Wijnhoven BPL, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 2000; 87:992 - 1005; http://dx.doi.org/10.1046/j.1365-2168.2000.01513.x; PMID: 10931041
- Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, et al. MALAT-1 a novel noncoding RNA, and thymosin beta 4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003; 22:8031 - 41; http://dx.doi.org/10.1038/sj.onc.1206928; PMID: 12970751
- Kobayashi T, Okada F, Fujii N, Tomita N, Ito S, Tazawa H, et al. Thymosin-beta 4 regulates motility and metastasis of malignant mouse fibrosarcoma clls. Am J Pathol 2002; 160:869 - 82; http://dx.doi.org/10.1016/S0002-9440(10)64910-3; PMID: 11891186
- Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y. Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene 2004; 23:6666 - 71; http://dx.doi.org/10.1038/sj.onc.1207888; PMID: 15235586
- Portyanko A, Kovalev P, Gorgun J, Cherstvoy E. βIII-tubulin at the invasive margin of colorectal cancer: possible link to invasion. Virchows Arch 2009; 454:541 - 8; http://dx.doi.org/10.1007/s00428-009-0764-4; PMID: 19360438
- Huang HC, Hu CH, Tang MC, Wang WS, Chen PM, Su Y. Thymosin β4 trigger an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 2007; 26:2781 - 90; http://dx.doi.org/10.1038/sj.onc.1210078; PMID: 17072345
- Smart N, Bollini S, Dubè KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011; 474:640 - 4; PMID: 21654746