Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has great potential for the treatment of cancer because it targets tumor cells while sparing normal cells. Several cancers, however, fail to respond to TRAIL's antineoplastic effects. These resistant tumors require cotreatment with sensitizing agents in order for TRAIL to exert anticancer activity. Histone deacetylase inhibitors (HDACi) have been recognized as potent TRAIL sensitizers. In searching for the determinants of TRAIL responsiveness, HDACi-mediated TRAIL sensitization has been predominantly attributed to TRAIL receptor upregulation. This explanation, however, has been challenged by a few studies. The aim of the present study was to explore the relevance of TRAIL receptor expression for HDACi-mediated TRAIL sensitization in childhood tumors, i.e., in medulloblastoma, Ewing's sarcoma and osteosarcoma. In previous studies, we had shown that TRAIL and HDACi were synergistic in inducing apoptosis in medulloblastoma and Ewing's sarcoma. In the present study, we demonstrate that HDACi cooperated with TRAIL in eliciting cell death in osteosarcoma. However, HDACi treatment did not alter or even reduced cell surface expression of TRAIL receptors in the three childhood tumors. In gaining insight into the apoptotic pathway involved in TRAIL sensitization, HDACi were found to potentiate TRAIL-induced caspase-8 activation. Taken together, our findings suggest that HDACi-mediated TRAIL sensitization is not the result of TRAIL receptor upregulation, but the result of a receptor-proximal event in childhood tumor cells.
Introduction
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL; also called Apo2L and TNFSF10) is a promising candidate for cancer treatment because it specifically kills malignant cells while leaving normal cells relatively unscathed. Consequently, clinical trials with recombinant TRAIL and agonistic anti-TRAIL receptor (TRAIL-R) monoclonal antibodies have been/are being conducted.Citation1 But these clinical trials have also revealed that many human tumors fail to respond to TRAIL monotherapy. TRAIL resistance, however, may be overcome by cotreatment with sensitizing agents, such as ionizing radiation or cytostatics. In particular, histone deacetylase inhibitors (HDACi), a promising new class of antineoplastic drugs that stimulate differentiation, induce apoptosis, inhibit proliferation and exert antiangiogenic and immune stimulatory activity in tumor cells,Citation2 have been documented in nearly 80 preclinical studies to enhance TRAIL's anticancer action.Citation3-Citation80
TRAIL instigates the extrinsic pathway of apoptosis by binding to its receptors TRAIL-R1 (also called DR4 and TNFRSF10A) or TRAIL-R2 (also called DR5 and TNFRSF10B), resulting in receptor oligomerization and the formation of a death-inducing signaling complex (DISC) consisting of activated receptors, the adaptor protein Fas-associated death domain (FADD) and caspase-8 or -10.Citation1 This leads to activation of caspase-8/10 by autocatalytic cleavage, which directly activates the executioner caspases 3 and 7. In addition, TRAIL can harness the intrinsic (mitochondrial) pathway of apoptosis: activated caspase-8/10 processes the Bcl-2 family protein Bid into a truncated form that translocates to the mitochondria, promoting the release of proapoptotic factors into the cytosol. This results in the formation of the apoptosome consisting of cytochrome c, Apaf-1 and caspase-9, in turn stimulating the activation of caspases 3 and 7. The apoptotic machinery is negatively regulated at several levels by antiapoptotic factors. For instance, c-FLIP interacts with the DISC and prevents caspase-8/10 activation, antiapoptotic Bcl-2 family members, such as Bcl-2, Bcl-xL, Mcl-1 and A1, block mitochondrial events, and inhibitor of apoptosis proteins (IAPs) inhibit activated caspases.Citation1
A conglomeration of mechanisms have been put forward to account for HDACi-mediated TRAIL sensitization, among them downregulation of c-FLIPCitation3,Citation8,Citation13,Citation23-Citation26,Citation32,Citation49,Citation52,Citation54,Citation59,Citation63,Citation67,Citation71,Citation73,Citation80 as well as ubiquitin/proteasome-mediated degradation of c-FLIP,Citation70,Citation78 enhanced recruitment of FADD,Citation66 facilitated formation of the DISC,Citation12 downregulation of Bcl-2,Citation8,Citation21,Citation23,Citation25,Citation39,Citation52,Citation60,Citation73,Citation75 Bcl-xL,Citation8,Citation21,Citation23-Citation25,Citation39,Citation40,Citation45,Citation52,Citation58,Citation60,Citation73,Citation75 Mcl-1Citation25,Citation40,Citation45 and A1,Citation23 upregulation of the proapoptotic Bcl-2 family proteins Bax and Bak,Citation21,Citation25,Citation40,Citation60,Citation75 upregulation of BidCitation11 as well as enhanced cleavage of Bid,Citation6,Citation45,Citation55,Citation69 upregulation of the proapoptotic “BH3-only” Bcl-2 family members Bim,Citation21,Citation25,Citation39,Citation40,Citation60 Noxa and PUMA,Citation21,Citation25,Citation60 downregulation of the IAPs XIAPCitation8,Citation20,Citation21,Citation25,Citation40,Citation45,Citation73 and survivinCitation8,Citation20,Citation73,Citation79 as well as degradation of XIAP,Citation57 activation of caspase-2,Citation44 upregulation of Apaf-1,Citation72 downregulation of the antiapoptotic transcription factor NF-κB,Citation60,Citation75,Citation77 modulation of NF-κB activityCitation18,Citation19,Citation28 as well as cleavage of NF-κB,Citation63,Citation68 cleavage of the prosurvival protein kinase Akt,Citation63 inhibition of the EGFR pathway,Citation59 downregulation of the Bcr-Abl fusion protein in chronic myeloid leukemia,Citation58 and, in conjunction with either IFNγ or decitabine, restoration of epigenetically silenced caspase-8 expression.Citation62,Citation79
But most prominently, HDACi-mediated TRAIL sensitization has been attributed to upregulation of TRAIL-R1 and/or -R2.Citation8-Citation11,Citation15,Citation17-Citation19,Citation21,Citation22,Citation25,Citation27,Citation29,Citation31,Citation37,Citation45,Citation46,Citation52,Citation56,Citation60,Citation61,Citation63,Citation64,Citation68,Citation69,Citation72,Citation74 Several studies, however, have found no association of enhanced TRAIL susceptibility with increased TRAIL-R expression.Citation6,Citation12,Citation32,Citation34,Citation38,Citation47,Citation54,Citation55,Citation73 Hence, in spite of extensive research into the combination of TRAIL and HDACi, the relevance of TRAIL-R expression has not been conclusively resolved. To shed more light on this issue—with an emphasis on childhood tumors—we examined HDACi effects on TRAIL-R expression in cell lines derived from medulloblastoma, Ewing's sarcoma and osteosarcoma. We found that HDACi treatment did not upregulate TRAIL-R cell surface expression, but did facilitate TRAIL-induced caspase-8 activation.
Results
Vorinostat and TRAIL synergize in inducing antineoplastic activity in osteosarcoma cells
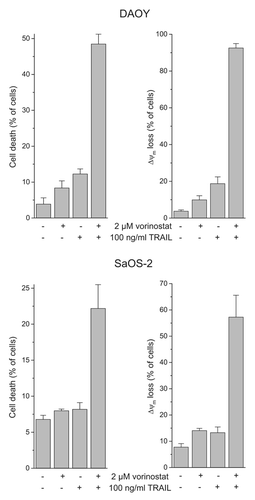
We showed previously that HDACi and TRAIL synergized to elicit cell death in medulloblastoma and Ewing's sarcoma cells.Citation30,Citation47 To confirm these observations, we reassessed the HDACi/TRAIL combination treatment in DAOY medulloblastoma cells. Cell death and Δψm dissipation were determined by flow cytometric analyses of propidium iodide uptake and DiOC6(3) staining, respectively. Four hours after treatment with the HDACi vorinostat, cells were exposed to TRAIL for another 48 h. As presented in , DAOY cells showed some responsiveness to vorinostat or TRAIL alone, but strong responsiveness to their combined application. To test a possible favorable cytotoxic interaction between HDACi and TRAIL in osteosarcoma cells, we examined cell death and Δψm dissipation in SaOS-2 cells. Either of the agents had little effect on the osteosarcoma cells when applied individually, but when applied together, they induced both considerable cell death and mitochondrial depolarization.
Figure 1. Vorinostat and TRAIL cooperate in inducing cell death and Δψm loss in DAOY and SaOS-2 cells. Four hours after administration of vorinostat, cells were exposed to TRAIL for another 48 h. Cell death and Δψm dissipation were determined by flow cytometric analyses of propidium iodide uptake and DiOC6(3) staining, respectively. Means ± SEM of each three separate experiments are shown.
HDACi do not upregulate surface expression of TRAIL-R in childhood tumor cells
In former studies on the combination of HDACi and TRAIL in childhood malignancies, we could not observe HDACi to induce an increase in TRAIL-R expression, neither in Ewing's sarcoma nor in hepatocellular carcinoma or hepatoblastoma cells.Citation47,Citation55 In the present study, we extended the investigation into the relevance of TRAIL-R expression for HDACi-mediated TRAIL sensitization to medulloblastoma and osteosarcoma. To begin with, we re-evaluated the effect of HDACi on TRAIL-R2 expression in WE-68 Ewing's sarcoma cells. We applied HDACi belonging to three different structural classes, the hydroxamic acid vorinostat, the cyclic tetrapeptide apicidin and the benzamide MS-275, and determined cell surface receptor expression by flow cytometric detection using monoclonal antibodies. Treatment with vorinostat or apicidin caused a significant decrease in TRAIL-R2 expression and MS-275 treatment produced a trend toward reduced expression of TRAIL-R2 (). We also assessed the effects of TRAIL and vorinostat/TRAIL treatment on TRAIL-R2 expression. TRAIL alone caused a trend toward reduced TRAIL-R2 expression, while the combination of vorinostat and TRAIL had a significant effect. As a comparison, we determined the effects of the anticancer drugs etoposide and bortezomib, which have been shown to upregulate TRAIL-R2.Citation19,Citation81 Etoposide treatment significantly increased TRAIL-R2 expression, whereas bortezomib treatment produced no significant effect.
Figure 2. HDACi do not upregulate TRAIL-R cell surface expression in childhood tumor cells. Cells were treated with agents for 24 h or for the indicated times and stained with TRAIL-R antibodies or isotype control. TRAIL-R cell surface expression was determined by flow cytometric analysis of viable cells. TRAIL-R mean fluorescence is depicted as percentage of untreated cells after calculation of the mean fluorescence intensities of anti-TRAIL-R-stained cells divided by isotype-matched IgG-stained cells; means ± SEM of each 3 experiments are shown (treatment vs. control: *p < 0.05, **p < 0.01; vorinostat/TRAIL vs. TRAIL: ##p < 0.01).
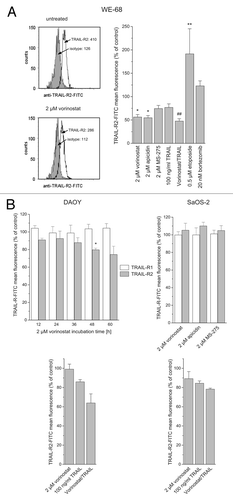
Next, we determined expression of TRAIL-R1 and -R2 in DAOY medulloblastoma and SaOS-2 osteosarcoma cells. DAOY cells were treated with 2 µM vorinostat—a treatment that strongly enhances TRAIL-induced cell death in DAOY cells (see )—and receptor levels were measured at 12 h intervals over a time course of 60 h. shows that TRAIL-R1 expression remained unchanged, while TRAIL-R2 expression slightly decreased. SaOS-2 cells were exposed to vorinostat, apicidin or MS-275 for 24 h: all three HDACi failed to increase TRAIL-R1 and -R2 levels. In DAOY and SaOS-2 cells, neither TRAIL alone nor the combination of vorinostat and TRAIL did have a significant effect on TRAIL-R2 expression (though a trend toward TRAIL-R2 downregulation could be observed after vorinostat/TRAIL combination treatment in DAOY cells).
In several studies, the importance of elevated TRAIL-R expression for HDACi-mediated TRAIL sensitization has been inferred from increased expression of TRAIL-R mRNA and/or protein by western blotting.Citation8-Citation11,Citation19,Citation60,Citation63,Citation64,Citation69,Citation74 However, HDACi have been found to upregulate TRAIL-R mRNA without concomitant change of TRAIL-R cell surface expression,Citation39 and, in Ewing's sarcoma cells, expression of TRAIL-R by western blotting did not strictly correlate with TRAIL-R surface expression.Citation82 To elucidate whether HDACi upregulate TRAIL-R2 mRNA in the childhood cancer cells, we conducted real-time RT-PCR analyses. As depicted in 24 h incubation with 2 µM vorinostat produced inconsistent results: TRAIL-R2 mRNA was upregulated in DAOY cells, but downregulated in WE-68 cells. Hence, the HDACi effect on TRAIL-R2 mRNA expression did not regularly match that on TRAIL-R2 surface expression. By comparison, etoposide induced TRAIL-R2 mRNA upregulation, in keeping with its effect on TRAIL-R2 cell surface expression.
Figure 3. Vorinostat alters TRAIL-R2 and c-FLIP mRNA expression in childhood tumor cells. Cells were exposed to agents for 24 h. TRAIL-R2 and c-FLIP mRNA expression levels were determined by real-time RT-PCR and normalized to β-2-microglobulin mRNA levels. Means ± SEM of each two separate measurements are shown (*p < 0.05, **p < 0.01).
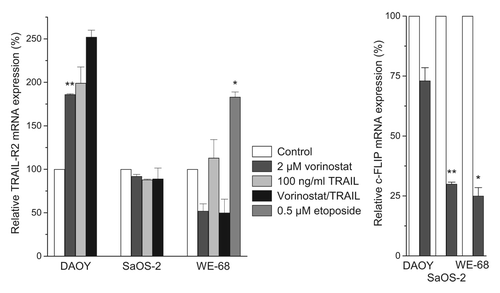
Next to TRAIL-R upregulation, HDACi-mediated TRAIL sensitization has been attributed most commonly to c-FLIP downregulation.Citation3,Citation8,Citation13,Citation23-Citation26,Citation32,Citation49,Citation52,Citation54,Citation59,Citation63,Citation67,Citation71,Citation73,Citation80 We assessed c-FLIP mRNA expression by real-time RT-PCR in the childhood cancer cells. We found vorinostat treatment to affect c-FLIP gene expression only slightly in DAOY cells, but to substantially decrease it in SaOS-2 and WE-68 cells.
Vorinostat enhances TRAIL-induced caspase-8 activity
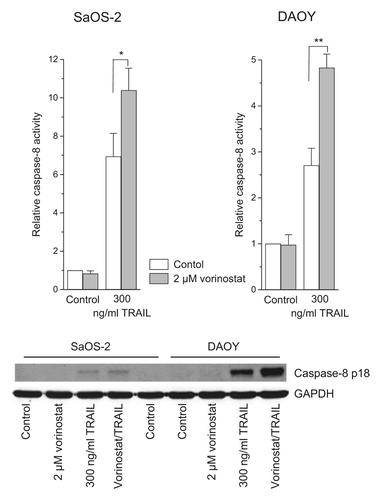
The data presented demonstrate that TRAIL-R upregulation was not essential for HDACi-mediated TRAIL sensitization in childhood tumor cells. To find out whether sensitization occurs before bifurcation into the mitochondria-dependent and -independent arm of apoptosis, we tested DAOY and SaOS-2 cells for activation of the receptor-proximal caspase-8. Caspase-8 activation was examined by measuring its catalytic activity using the fluorogenic substrate Ac-IETD-AMC and by detecting the cleaved subunit p18 on western blot. Four hours after treatment with vorinostat, cells were exposed to TRAIL for another 2 h. Both read-outs show that vorinostat augmented TRAIL-induced caspase-8 activition (), suggesting that HDACi potentiate TRAIL-elicited apoptosis at the level of the DISC.
Figure 4. Vorinostat potentiates TRAIL-induced caspase-8 activity in childhood tumor cells. Four hours after administration of vorinostat, cells were exposed to TRAIL for another 2 h. Caspase-8 activity was determined using the fluorogenic substrate Ac-IETD-AFC; relative caspase-8 activities are the ratio of treated cells to untreated cells. Means ± SEM of each 3 separate measurements are shown (*p < 0.05, **p < 0.01). Caspase-8 cleavage, i.e., the subunit p18, was detected by immunoblotting using anti-cleaved caspase-8 antibody.
Discussion
Almost 80 studies have demonstrated that HDACi can enhance the anticancer activity of TRAIL.Citation3-Citation80 Of them, more than 20 have proposed that HDACi-mediated TRAIL sensitization primarily stems from increased TRAIL-R expression.Citation8-Citation11,Citation15,Citation17-Citation19,Citation21,Citation22,Citation25,Citation27,Citation29,Citation31,Citation37,Citation45,Citation46,Citation52,Citation56,Citation60,Citation61,Citation63,Citation64,Citation68,Citation69,Citation72,Citation74 Yet a few have challenged the relevance of TRAIL-R upregulation.Citation6,Citation12,Citation32,Citation38,Citation47,Citation54,Citation55,Citation73 In fact, HDACi have been observed to sensitize to agonistic TRAIL-R2 antibodies even in the presence of decreased TRAIL-R2 expression.Citation34 Our study lends further support to the insignificance of TRAIL-R expression. In particular, it argues against a role of TRAIL-R expression in HDACi-mediated TRAIL sensitization in childhood malignancies. We have shown that HDACi synergized with TRAIL in medulloblastoma,Citation30 in Ewing's sarcomaCitation47 and in osteosarcoma (this study), but did not upregulate - actually rather downregulated - TRAIL-R in these cells. This conclusion is also backed by our former study on childhood hepatocellular carcinoma and hepatoblastoma, in which HDACi synergized with TRAIL without upregulating TRAIL-R.Citation55
Our data presented here, however, indicate that HDACi-mediated TRAIL sensitization is a receptor-proximal event in childhood tumor cells. We found HDACi treatment to significantly decrease c-FLIP gene expression and, more importantly, to enhance TRAIL-induced caspase-8 activation. In principle, enhanced caspase-8 activity could also be the result of feedback effects of downstream executioner caspases.Citation1 Yet, the activity of caspase-8 was measured at 2 h post-addition of TRAIL—in fact, some increase in caspase-8 activity was observed as early as one hour after TRAIL administration (not shown)—arguing against the possibility that the potentiated activation of caspase-8 was secondary to HDACi-amplified activation of executioner caspases. This result is in line with our former study on childhood hepatoma cells showing that HDACi second TRAIL in promoting Bid cleavageCitation55 and it is also in line with a former study on leukemia cells showing that HDACi facilitate DISC formation and caspase-8 activation in the absence of TRAIL-R upregulation.Citation12
For comparison, we also assessed the effect of the proteasome inhibitor bortezomib on TRAIL-R2 expression in Ewing's sarcoma cells. In a study on the combination of TRAIL with diverse anticancer agents, bortezomib has been reported to be the most effective one at overcoming TRAIL resistance.Citation76 As with HDACi, the role of TRAIL-R expression in bortezomib-mediated TRAIL sensitization has not been unambiguously unraveled: upregulation of TRAIL-R2 has both been reported to be requiredCitation81 and to be not requiredCitation83 for bortezomib-mediated sensitization to TRAIL. Bortezomib has previously been demonstrated to synergize with TRAIL in Ewing's sarcoma.Citation84 Here, we have shown that bortezomib treatment only led to an insignificant increase in TRAIL-R2 expression, indicating that its upregulation is not critical for sensitization to TRAIL, at least in Ewing's sarcoma. This finding taken together with the results of the HDACi treatments suggests that TRAIL-R expression in general is not a suitable predictor for TRAIL susceptibility in childhood cancer. Support for this conclusion also comes from a study on the primary responsiveness of Ewing's sarcoma to TRAIL, in which no difference in the TRAIL-R surface expression was observed between sensitive and resistant cells.Citation85
In fact, key determinants, if any, of HDACi-mediated TRAIL sensitization are far from being consistently defined (see Introduction). Rather, the plethora of mechanisms reported to participate in HDACi-mediated TRAIL sensitization raise the question if a single key determinant of TRAIL responsiveness exists. In single-cell analysis of TRAIL-induced apoptosis, TRAIL susceptibility was not governed by the expression level of any individual protein, but by expression variations of multiple proteins together.Citation86 Fortunately, the effects of HDACi go beyond the modulation of single protein levelsCitation2 and, thus, they have the potential to overcome resistance, even if the latter is not the result of unfavorable expression of any one protein in the apoptotic machinery, such as TRAIL-R.
In conclusion, our data demonstrate that TRAIL-R upregulation is not critical for HDACi to render childhood tumors susceptible to TRAIL. They also indicate that HDACi-mediated TRAIL sensitization is a receptor-proximal event, i.e., the consequence of hyperactivation of the extrinsic pathway, though we cannot rule out the possibility that HDACi boost TRAIL-induced apoptosis in addition by activating the intrinsic pathway.
Materials and Methods
Reagents
Vorinostat, apicidin, MS-275 and etoposide were purchased from Alexis. TRAIL was purchased from Peprotech. Bortezomib was purchased from LC Laboratories.
Cell lines
WE-68 cells (provided by Dr. F. van Valen, Münster, Germany) and SaOS-2 cells (a gift from Dr. T. Beckers, Konstanz, Germany) were maintained in RPMI 1640 (PAA) and DAOY cells (a gift from Dr. M. Grotzer, Zurich, Switzerland) were maintained in Improved MEM Zinc Option (Invitrogen). Media were supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 units/ml penicillin G sodium and 100 µg/ml streptomycin sulfate (PAA). WE-68 cells were cultivated in collagen-coated (5 µg/cm2; Roche) tissue culture flasks. Cells were cultivated at 37°C in a humidified 5% CO2 incubator and routinely passaged when 90% confluent. Cell viability was determined by the trypan blue exclusion test. Cells were regularly inspected to be free of mycoplasma with the PCR mycoplasma detection kit from Applichem.
Flow cytometric analysis of cell death and mitochondrial transmembrane potential (Δψm)
Cell death was assessed by determining the integrity of the cell membrane by flow cytometric analysis of propidium iodide (Sigma) uptake. After harvesting, cells were incubated for 5 min in 2 µg/ml propidium iodide in PBS at 4°C in the dark. Δψm was assessed by determining the accumulation of 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] (Molecular Probes) in the mitochondrial matrix. Before harvesting, cells were incubated with 50 nM DiOC6(3) at 37°C for 30 min. In both readouts, 10,000 cells were analyzed in each sample on a BD FACSCanto II; data were gated to exclude debris.
Flow cytometric detection of TRAIL-R1 and -R2 cell surface expression
Cells were harvested with 0.2% EDTA, washed twice with PBS, and incubated with mouse anti-TRAIL-R1/2 monoclonal antibodies (2 µg in 100 µl PBS/2.5% BSA; BioLegend) for 30 min at ambient temperature in the dark. Cells were washed twice and stained with a FITC-conjugated goat anti-mouse IgG antibody (20 µg/ml in 2.5% BSA; Dianova) for 30 min at ambient temperature in the dark. After washing, 50,000 cells were analyzed using the FACSCanto II; cells were gated on viable cells. A mouse IgG (20 µg/ml in 2.5% BSA; eBioscience) was used as isotype-matched control. Frequency histograms were generated using Flowjo software. Data are shown as fold induction after calculation of the mean fluorescence intensities of anti-TRAIL-R1/2-stained cells divided by isotype-matched IgG-stained cells.
Quantitative real-time RT-PCR
Total RNA was isolated using Peqgold Total RNA Kit including DNase digestion (Peqlab). RNA was transcribed into cDNA using Omniscript (Qiagen). Quantitative PCR for TRAIL-R2 was performed using the 7900HT Real-Time PCR system (Applied Biosystems). Expression levels were normalized to β-2-microglobulin. Reactions were done in duplicate using Applied Biosystems Gene Expression Assays (TRAIL-R2: Hs00187196_m1; c-FLIP: Hs01116280_m1; β-2-microglobulin: Hs00187842_m1) and Universal PCR Master Mix. All procedures were performed according to the manufacturers' protocols. The relative TRAIL-R2 expression was calculated by the 2(-ΔΔCt) method.
Caspase-8 activity and cleavage
Caspase-8 activity was measured using the fluorogenic substrate Ac-IETD-AMC (Bachem). After harvesting, cells were lysed in 10 mM TRIS-HCl, 10 mM NaH2PO4/NaHPO4 (pH 7.5), 130 mM NaCl, 1% Triton X-100 and 10 mM Na4P2O7 and then incubated with 20 mM Hepes (pH 7.5), 10% glycerol, 2 mM DTT, 10 µM MG-132 (in order to reduce nonspecific background by inhibiting the caspase-like activity of the proteasome) and 25 µg/ml Ac-IETD-AMC at 37°C for 2 h. The release of AMC was measured on a BMG Labtech FLUOstar Omega using an excitation/emission wavelength of 355/460 nm. Relative caspase-8 activities were calculated as a ratio of emission of treated cells to untreated cells.
Caspase-8 cleavage was detected by immunoblotting. 40 µg of total cellular protein per lane were separated by standard SDS-PAGE on 12% gel and electrophoretically transferred to nitrocellulose membrane. After blocking, caspase-8 cleavage was immunodetected using rabbit anti-cleaved caspase-8 (18C8) monoclonal antibody (dilution 1:1,000; #9496, Cell Signaling). Equal loading of protein was verified by detection of GAPDH using mouse anti-GAPDH monoclonal antibody (dilution 1:25,000; Biodesign International). Peroxidase-conjugated goat anti-rabbit IgG (dilution 1:5,000; Dianova) and goat anti-mouse IgG (dilution 1:25,000; Dianova) were used as secondary antibodies followed by enhanced chemiluminescence detection (GE Healthcare) of specific signals.
Statistical analysis
Statistical significance of differences between experimental groups was determined using the paired two-tailed Student's t test.
| Abbreviations: | ||
| DiOC6(3) | = | 3,3′-dihexyloxacarbocyanine iodide |
| DISC | = | death-inducing signaling complex |
| HDACi | = | histone deacetylase inhibitors |
| TRAIL-R | = | TRAIL receptor |
Acknowledgments
This work was supported by a grant from the “Wilhelm Sander-Stiftung, Neustadt/Donau.”
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov 2008; 7:1001 - 12; http://dx.doi.org/10.1038/nrd2637; PMID: 18989337
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 2009; 27:5459 - 68; http://dx.doi.org/10.1200/JCO.2009.22.1291; PMID: 19826124
- Hernandez A, Thomas R, Smith F, Sandberg J, Kim S, Chung DH, et al. Butyrate sensitizes human colon cancer cells to TRAIL-mediated apoptosis. Surgery 2001; 130:265 - 72; http://dx.doi.org/10.1067/msy.2001.115897; PMID: 11490359
- Inoue H, Shiraki K, Ohmori S, Sakai T, Deguchi M, Yamanaka T, et al. Histone deacetylase inhibitors sensitize human colonic adenocarcinoma cell lines to TNF-related apoptosis inducing ligand-mediated apoptosis. Int J Mol Med 2002; 9:521 - 5; PMID: 11956660
- Mitsiades N, Mitsiades CS, Richardson PG, McMullan C, Poulaki V, Fanourakis G, et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood 2003; 101:4055 - 62; http://dx.doi.org/10.1182/blood-2002-11-3514; PMID: 12531799
- Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther 2003; 2:1273 - 84; PMID: 14707268
- Neuzil J, Swettenham E, Gellert N. Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of Bcl-xL down-regulation. Biochem Biophys Res Commun 2004; 314:186 - 91; http://dx.doi.org/10.1016/j.bbrc.2003.12.074; PMID: 14715264
- Guo F, Sigua C, Tao J, Bali P, George P, Li Y, et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res 2004; 64:2580 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-03-2629; PMID: 15059915
- Kim YH, Park JW, Lee JY, Kwon TK. Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells. Carcinogenesis 2004; 25:1813 - 20; http://dx.doi.org/10.1093/carcin/bgh188; PMID: 15142888
- Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene 2004; 23:6261 - 71; http://dx.doi.org/10.1038/sj.onc.1207830; PMID: 15208660
- Chopin V, Slomianny C, Hondermarck H, Le Bourhis X. Synergistic induction of apoptosis in breast cancer cells by cotreatment with butyrate and TNF-alpha, TRAIL, or anti-Fas agonist antibody involves enhancement of death receptors’ signaling and requires P21(waf1). Exp Cell Res 2004; 298:560 - 73; http://dx.doi.org/10.1016/j.yexcr.2004.04.038; PMID: 15265702
- Inoue S, MacFarlane M, Harper N, Wheat LM, Dyer MJ, Cohen GM. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ 2004; 11:Suppl 2 S193 - 206; http://dx.doi.org/10.1038/sj.cdd.4401535; PMID: 15608694
- Watanabe K, Okamoto K, Yonehara S. Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. Cell Death Differ 2005; 12:10 - 8; http://dx.doi.org/10.1038/sj.cdd.4401507; PMID: 15540114
- Sonnemann J, Gänge J, Kumar KS, Müller C, Bader P, Beck JF. Histone deacetylase inhibitors interact synergistically with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to induce apoptosis in carcinoma cell lines. Invest New Drugs 2005; 23:99 - 109; http://dx.doi.org/10.1007/s10637-005-5854-9; PMID: 15744585
- Vanoosten RL, Moore JM, Ludwig AT, Griffith TS. Depsipeptide (FR901228) enhances the cytotoxic activity of TRAIL by redistributing TRAIL receptor to membrane lipid rafts. Mol Ther 2005; 11:542 - 52; http://dx.doi.org/10.1016/j.ymthe.2004.12.008; PMID: 15771957
- Taghiyev AF, Guseva NV, Sturm MT, Rokhlin OW, Cohen MB. Trichostatin A (TSA) sensitizes the human prostatic cancer cell line DU145 to death receptor ligands treatment. Cancer Biol Ther 2005; 4:382 - 90; http://dx.doi.org/10.4161/cbt.4.4.1615; PMID: 15846101
- MacFarlane M, Inoue S, Kohlhaas SL, Majid A, Harper N, Kennedy DB, et al. Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death Differ 2005; 12:773 - 82; http://dx.doi.org/10.1038/sj.cdd.4401649; PMID: 15861184
- Singh TR, Shankar S, Srivastava RK. HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene 2005; 24:4609 - 23; http://dx.doi.org/10.1038/sj.onc.1208585; PMID: 15897906
- Shetty S, Graham BA, Brown JG, Hu X, Vegh-Yarema N, Harding G, et al. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol Cell Biol 2005; 25:5404 - 16; http://dx.doi.org/10.1128/MCB.25.13.5404-5416.2005; PMID: 15964798
- Kim EH, Kim HS, Kim SU, Noh EJ, Lee JS, Choi KS. Sodium butyrate sensitizes human glioma cells to TRAIL-mediated apoptosis through inhibition of Cdc2 and the subsequent downregulation of survivin and XIAP. Oncogene 2005; 24:6877 - 89; http://dx.doi.org/10.1038/sj.onc.1208851; PMID: 16007142
- Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK. Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia 2005; 7:646 - 57; http://dx.doi.org/10.1593/neo.04655; PMID: 16026644
- VanOosten RL, Moore JM, Karacay B, Griffith TS. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L-induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol Ther 2005; 4:1104 - 12; http://dx.doi.org/10.4161/cbt.4.10.2022; PMID: 16096370
- Mitsiades CS, Poulaki V, McMullan C, Negri J, Fanourakis G, Goudopoulou A, et al. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res 2005; 11:3958 - 65; http://dx.doi.org/10.1158/1078-0432.CCR-03-0776; PMID: 15897598
- El-Zawahry A, Lu P, White SJ, Voelkel-Johnson C. In vitro efficacy of AdTRAIL gene therapy of bladder cancer is enhanced by trichostatin A-mediated restoration of CAR expression and downregulation of cFLIP and Bcl-XL. Cancer Gene Ther 2006; 13:281 - 9; http://dx.doi.org/10.1038/sj.cgt.7700905; PMID: 16167063
- Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: involvement of both death receptor and mitochondrial pathways. Int J Mol Med 2005; 16:1125 - 38; PMID: 16273296
- Schuchmann M, Schulze-Bergkamen H, Fleischer B, Schattenberg JM, Siebler J, Weinmann A, et al. Histone deacetylase inhibition by valproic acid down-regulates c-FLIP/CASH and sensitizes hepatoma cells towards CD95- and TRAIL receptor-mediated apoptosis and chemotherapy. Oncol Rep 2006; 15:227 - 30; PMID: 16328060
- Earel JK Jr., VanOosten RL, Griffith TS. Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res 2006; 66:499 - 507; http://dx.doi.org/10.1158/0008-5472.CAN-05-3017; PMID: 16397266
- Lakshmikanthan V, Kaddour-Djebbar I, Lewis RW, Kumar MV. SAHA-sensitized prostate cancer cells to TNFalpha-related apoptosis-inducing ligand (TRAIL): mechanisms leading to synergistic apoptosis. Int J Cancer 2006; 119:221 - 8; http://dx.doi.org/10.1002/ijc.21824; PMID: 16450389
- VanOosten RL, Earel JK Jr., Griffith TS. Enhancement of Ad5-TRAIL cytotoxicity against renal cell carcinoma with histone deacetylase inhibitors. Cancer Gene Ther 2006; 13:628 - 32; http://dx.doi.org/10.1038/sj.cgt.7700939; PMID: 16456549
- Sonnemann J, Kumar KS, Heesch S, Müller C, Hartwig C, Maass M, et al. Histone deacetylase inhibitors induce cell death and enhance the susceptibility to ionizing radiation, etoposide, and TRAIL in medulloblastoma cells. Int J Oncol 2006; 28:755 - 66; PMID: 16465382
- Butler LM, Liapis V, Bouralexis S, Welldon K, Hay S, Thai M, et al. The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL. Int J Cancer 2006; 119:944 - 54; http://dx.doi.org/10.1002/ijc.21939; PMID: 16550602
- Pathil A, Armeanu S, Venturelli S, Mascagni P, Weiss TS, Gregor M, et al. HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology 2006; 43:425 - 34; http://dx.doi.org/10.1002/hep.21054; PMID: 16583461
- Thai M, Labrinidis A, Hay S, Liapis V, Bouralexis S, Welldon K, et al. Apo2l/Tumor necrosis factor-related apoptosis-inducing ligand prevents breast cancer-induced bone destruction in a mouse model. Cancer Res 2006; 66:5363 - 70; http://dx.doi.org/10.1158/0008-5472.CAN-05-4386; PMID: 16707463
- Inoue S, Twiddy D, Dyer MJ, Cohen GM. Upregulation of TRAIL-R2 is not involved in HDACi mediated sensitization to TRAIL-induced apoptosis. Cell Death Differ 2006; 13:2160 - 2; http://dx.doi.org/10.1038/sj.cdd.4401977; PMID: 16729023
- Inoue S, Mai A, Dyer MJ, Cohen GM. Inhibition of histone deacetylase class I but not class II is critical for the sensitization of leukemic cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Cancer Res 2006; 66:6785 - 92; http://dx.doi.org/10.1158/0008-5472.CAN-05-4563; PMID: 16818655
- Ziauddin MF, Yeow WS, Maxhimer JB, Baras A, Chua A, Reddy RM, et al. Valproic acid, an antiepileptic drug with histone deacetylase inhibitory activity, potentiates the cytotoxic effect of Apo2L/TRAIL on cultured thoracic cancer cells through mitochondria-dependent caspase activation. Neoplasia 2006; 8:446 - 57; http://dx.doi.org/10.1593/neo.05823; PMID: 16820090
- Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res 2006; 66:7317 - 25; http://dx.doi.org/10.1158/0008-5472.CAN-06-0680; PMID: 16849582
- Mühlethaler-Mottet A, Flahaut M, Bourloud KB, Auderset K, Meier R, Joseph JM, et al. Histone deacetylase inhibitors strongly sensitise neuroblastoma cells to TRAIL-induced apoptosis by a caspases-dependent increase of the pro- to anti-apoptotic proteins ratio. BMC Cancer 2006; 6:214; http://dx.doi.org/10.1186/1471-2407-6-214; PMID: 16930472
- Fandy TE, Srivastava RK. Trichostatin A sensitizes TRAIL-resistant myeloma cells by downregulation of the antiapoptotic Bcl-2 proteins. Cancer Chemother Pharmacol 2006; 58:471 - 7; http://dx.doi.org/10.1007/s00280-005-0184-3; PMID: 16435155
- Gillespie S, Borrow J, Zhang XD, Hersey P. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis 2006; 11:2251 - 65; http://dx.doi.org/10.1007/s10495-006-0283-6; PMID: 17051334
- Kasman L, Lu P, Voelkel-Johnson C. The histone deacetylase inhibitors depsipeptide and MS-275, enhance TRAIL gene therapy of LNCaP prostate cancer cells without adverse effects in normal prostate epithelial cells. Cancer Gene Ther 2007; 14:327 - 34; http://dx.doi.org/10.1038/sj.cgt.7701017; PMID: 17186014
- Reddy RM, Yeow WS, Chua A, Nguyen DM, Baras A, Ziauddin MF, et al. Rapid and profound potentiation of Apo2L/TRAIL-mediated cytotoxicity and apoptosis in thoracic cancer cells by the histone deacetylase inhibitor Trichostatin A: the essential role of the mitochondria-mediated caspase activation cascade. Apoptosis 2007; 12:55 - 71; http://dx.doi.org/10.1007/s10495-006-0484-z; PMID: 17136498
- Garofalo M, Romano G, Quintavalle C, Romano MF, Chiurazzi F, Zanca C, et al. Selective inhibition of PED protein expression sensitizes B-cell chronic lymphocytic leukaemia cells to TRAIL-induced apoptosis. Int J Cancer 2007; 120:1215 - 22; http://dx.doi.org/10.1002/ijc.22495; PMID: 17192900
- VanOosten RL, Earel JK Jr., Griffith TS. Histone deacetylase inhibitors enhance Ad5-TRAIL killing of TRAIL-resistant prostate tumor cells through increased caspase-2 activity. Apoptosis 2007; 12:561 - 71; http://dx.doi.org/10.1007/s10495-006-0009-9; PMID: 17195089
- Lillehammer T, Engesaeter BO, Prasmickaite L, Maelandsmo GM, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J Gene Med 2007; 9:440 - 51; http://dx.doi.org/10.1002/jgm.1036; PMID: 17410615
- Gómez-Benito M, Martinez-Lorenzo MJ, Anel A, Marzo I, Naval J. Membrane expression of DR4, DR5 and caspase-8 levels, but not Mcl-1, determine sensitivity of human myeloma cells to Apo2L/TRAIL. Exp Cell Res 2007; 313:2378 - 88; http://dx.doi.org/10.1016/j.yexcr.2007.03.018; PMID: 17462628
- Sonnemann J, Dreyer L, Hartwig M, Palani CD, Hong TT, Klier U, et al. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma cells. J Cancer Res Clin Oncol 2007; 133:847 - 58; http://dx.doi.org/10.1007/s00432-007-0227-8; PMID: 17486365
- Nawrocki ST, Carew JS, Douglas L, Cleveland JL, Humphreys R, Houghton JA. Histone deacetylase inhibitors enhance lexatumumab-induced apoptosis via a p21Cip1-dependent decrease in survivin levels. Cancer Res 2007; 67:6987 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-07-0812; PMID: 17638911
- Lagneaux L, Gillet N, Stamatopoulos B, Delforge A, Dejeneffe M, Massy M, et al. Valproic acid induces apoptosis in chronic lymphocytic leukemia cells through activation of the death receptor pathway and potentiates TRAIL response. Exp Hematol 2007; 35:1527 - 37; http://dx.doi.org/10.1016/j.exphem.2007.06.014; PMID: 17697742
- Inoue S, Walewska R, Dyer MJ, Cohen GM. Downregulation of Mcl-1 potentiates HDACi-mediated apoptosis in leukemic cells. Leukemia 2008; 22:819 - 25; http://dx.doi.org/10.1038/leu.2008.1; PMID: 18239621
- Stieglmaier J, Bremer E, Kellner C, Liebig TM, ten Cate B, Peipp M, et al. Selective induction of apoptosis in leukemic B-lymphoid cells by a CD19-specific TRAIL fusion protein. Cancer Immunol Immunother 2008; 57:233 - 46; http://dx.doi.org/10.1007/s00262-007-0370-8; PMID: 17665197
- Iacomino G, Medici MC, Russo GL. Valproic acid sensitizes K562 erythroleukemia cells to TRAIL/Apo2L-induced apoptosis. Anticancer Res 2008; 28:2A 855 - 64; PMID: 18507029
- Feng R, Ma H, Hassig CA, Payne JE, Smith ND, Mapara MY, et al. KD5170, a novel mercaptoketone-based histone deacetylase inhibitor, exerts antimyeloma effects by DNA damage and mitochondrial signaling. Mol Cancer Ther 2008; 7:1494 - 505; http://dx.doi.org/10.1158/1535-7163.MCT-08-0183; PMID: 18566220
- Frew AJ, Lindemann RK, Martin BP, Clarke CJP, Sharkey J, Anthony DA, et al. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci U S A 2008; 105:11317 - 22; http://dx.doi.org/10.1073/pnas.0801868105; PMID: 18685088
- Dzieran J, Beck JF, Sonnemann J. Differential responsiveness of human hepatoma cells versus normal hepatocytes to TRAIL in combination with either histone deacetylase inhibitors or conventional cytostatics. Cancer Sci 2008; 99:1685 - 92; http://dx.doi.org/10.1111/j.1349-7006.2008.00868.x; PMID: 18754884
- Aguilera DG, Das CM, Sinnappah-Kang ND, Joyce C, Taylor PH, Wen S, et al. Reactivation of death receptor 4 (DR4) expression sensitizes medulloblastoma cell lines to TRAIL. J Neurooncol 2009; 93:303 - 18; http://dx.doi.org/10.1007/s11060-008-9788-x; PMID: 19148581
- Symanowski J, Vogelzang N, Zawel L, Atadja P, Pass H, Sharma S. A histone deacetylase inhibitor LBH589 downregulates XIAP in mesothelioma cell lines which is likely responsible for increased apoptosis with TRAIL. J Thorac Oncol 2009; 4:149 - 60; http://dx.doi.org/10.1097/JTO.0b013e318194f991; PMID: 19179889
- Park SJ, Kim MJ, Kim HB, Sohn HY, Bae JH, Kang CD, et al. Cotreatment with apicidin overcomes TRAIL resistance via inhibition of Bcr-Abl signaling pathway in K562 leukemia cells. Exp Cell Res 2009; 315:1809 - 18; http://dx.doi.org/10.1016/j.yexcr.2009.02.024; PMID: 19268463
- Park SJ, Kim MJ, Kim HB, Sohn HY, Bae JH, Kang CD, et al. Trichostatin A sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by down-regulation of c-FLIPL via inhibition of EGFR pathway. Biochem Pharmacol 2009; 77:1328 - 36; http://dx.doi.org/10.1016/j.bcp.2008.12.027; PMID: 19426671
- Shankar S, Davis R, Singh KP, Kurzrock R, Ross DD, Srivastava RK. Suberoylanilide hydroxamic acid (Zolinza/vorinostat) sensitizes TRAIL-resistant breast cancer cells orthotopically implanted in BALB/c nude mice. Mol Cancer Ther 2009; 8:1596 - 605; http://dx.doi.org/10.1158/1535-7163.MCT-08-1004; PMID: 19509267
- Norian LA, Kucaba TA, Earel JK, Knutson T, Vanoosten RL, Griffith TS. Synergistic induction of apoptosis in primary B-CLL cells after treatment with recombinant tumor necrosis factor-related apoptosis-inducing ligand and histone deacetylase inhibitors. J Oncol 2009; 2009:408038; http://dx.doi.org/10.1155/2009/408038; PMID: 19547714
- Häcker S, Dittrich A, Mohr A, Schweitzer T, Rutkowski S, Krauss J, et al. Histone deacetylase inhibitors cooperate with IFN-gamma to restore caspase-8 expression and overcome TRAIL resistance in cancers with silencing of caspase-8. Oncogene 2009; 28:3097 - 110; http://dx.doi.org/10.1038/onc.2009.161; PMID: 19597472
- Carlisi D, Lauricella M, D’Anneo A, Emanuele S, Angileri L, Di Fazio P, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Eur J Cancer 2009; 45:2425 - 38; http://dx.doi.org/10.1016/j.ejca.2009.06.024; PMID: 19643600
- Yeh CC, Deng YT, Sha DY, Hsiao M, Kuo MY. Suberoylanilide hydroxamic acid sensitizes human oral cancer cells to TRAIL-induced apoptosis through increase DR5 expression. Mol Cancer Ther 2009; 8:2718 - 25; http://dx.doi.org/10.1158/1535-7163.MCT-09-0211; PMID: 19737941
- Wood TE, Dalili S, Simpson CD, Sukhai MA, Hurren R, Anyiwe K, et al. Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. Mol Cancer Ther 2010; 9:246 - 56; http://dx.doi.org/10.1158/1535-7163.MCT-09-0495; PMID: 20053768
- Inoue S, Harper N, Walewska R, Dyer MJ, Cohen GM. Enhanced Fas-associated death domain recruitment by histone deacetylase inhibitors is critical for the sensitization of chronic lymphocytic leukemia cells to TRAIL-induced apoptosis. Mol Cancer Ther 2009; 8:3088 - 97; http://dx.doi.org/10.1158/1535-7163.MCT-09-0451; PMID: 19887558
- Darvas K, Rosenberger S, Brenner D, Fritsch C, Gmelin N, Krammer PH, et al. Histone deacetylase inhibitor-induced sensitization to TNFalpha/TRAIL-mediated apoptosis in cervical carcinoma cells is dependent on HPV oncogene expression. Int J Cancer 2010; 127:1384 - 92; http://dx.doi.org/10.1002/ijc.25170; PMID: 20087862
- D’Acunto CW, Carratù A, Rodriquez M, Taddei M, Parente L, Petrella A. LGP1, A histone deacetylase inhibitor analogue of FR235222, sensitizes promyelocytic leukaemia U937 cells to TRAIL-mediated apoptosis. Anticancer Res 2010; 30:887 - 94; PMID: 20393011
- Schüler S, Fritsche P, Diersch S, Arlt A, Schmid RM, Saur D, et al. HDAC2 attenuates TRAIL-induced apoptosis of pancreatic cancer cells. Mol Cancer 2010; 9:80; http://dx.doi.org/10.1186/1476-4598-9-80; PMID: 20398369
- Kauh J, Fan S, Xia M, Yue P, Yang L, Khuri FR, et al. c-FLIP degradation mediates sensitization of pancreatic cancer cells to TRAIL-induced apoptosis by the histone deacetylase inhibitor LBH589. PLoS One 2010; 5:e10376; http://dx.doi.org/10.1371/journal.pone.0010376; PMID: 20442774
- Lucas DM, Alinari L, West DA, Davis ME, Edwards RB, Johnson AJ, et al. The novel deacetylase inhibitor AR-42 demonstrates pre-clinical activity in B-cell malignancies in vitro and in vivo. PLoS One 2010; 5:e10941; http://dx.doi.org/10.1371/journal.pone.0010941; PMID: 20532179
- Morales JC, Ruiz-Magaña MJ, Carranza D, Ortiz-Ferrón G, Ruiz-Ruiz C. HDAC inhibitors with different gene regulation activities depend on the mitochondrial pathway for the sensitization of leukemic T cells to TRAIL-induced apoptosis. Cancer Lett 2010; 297:91 - 100; http://dx.doi.org/10.1016/j.canlet.2010.04.029; PMID: 20580868
- Sung ES, Kim A, Park JS, Chung J, Kwon MH, Kim YS. Histone deacetylase inhibitors synergistically potentiate death receptor 4-mediated apoptotic cell death of human T-cell acute lymphoblastic leukemia cells. Apoptosis 2010; 15:1256 - 69; http://dx.doi.org/10.1007/s10495-010-0521-9; PMID: 20582477
- Srivastava RK, Kurzrock R, Shankar S. MS-275 sensitizes TRAIL-resistant breast cancer cells, inhibits angiogenesis and metastasis, and reverses epithelial-mesenchymal transition in vivo. Mol Cancer Ther 2010; 9:3254 - 66; http://dx.doi.org/10.1158/1535-7163.MCT-10-0582; PMID: 21041383
- Martin BP, Frew AJ, Bots M, Fox S, Long F, Takeda K, et al. Antitumor activities and on-target toxicities mediated by a TRAIL receptor agonist following cotreatment with panobinostat. Int J Cancer 2011; 128:2735 - 47; http://dx.doi.org/10.1002/ijc.25594; PMID: 20715169
- Menke C, Bin L, Thorburn J, Behbakht K, Ford HL, Thorburn A. Distinct TRAIL resistance mechanisms can be overcome by proteasome inhibition but not generally by synergizing agents. Cancer Res 2011; 71:1883 - 92; http://dx.doi.org/10.1158/0008-5472.CAN-10-2252; PMID: 21363923
- Kim DR, Park MY, Lee CS, Shim SH, Yoon HI, Lee JH, et al. Combination of vorinostat and adenovirus-TRAIL exhibits a synergistic antitumor effect by increasing transduction and transcription of TRAIL in lung cancer cells. Cancer Gene Ther 2011; 18:467 - 77; http://dx.doi.org/10.1038/cgt.2011.11; PMID: 21455254
- Yerbes R, López-Rivas A. Itch/AIP4-independent proteasomal degradation of cFLIP induced by the histone deacetylase inhibitor SAHA sensitizes breast tumour cells to TRAIL. Invest New Drugs 2010; In press http://dx.doi.org/10.1007/s10637-010-9597-x; PMID: 21107885
- Kaminskyy VO, Surova OV, Vaculova A, Zhivotovsky B. Combined inhibition of DNA methyltransferase and histone deacetylase restores caspase-8 expression and sensitizes SCLC cells to TRAIL. Carcinogenesis 2011; 32:1450 - 8; http://dx.doi.org/10.1093/carcin/bgr135; PMID: 21771726
- Lauricella M, Ciraolo A, Carlisi D, Vento R, Tesoriere G. SAHA/TRAIL combination induces detachment and anoikis of MDA-MB231 and MCF-7 breast cancer cells. Biochimie 2011; In press PMID: 21835222
- Liu X, Yue P, Chen S, Hu L, Lonial S, Khuri FR, et al. The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res 2007; 67:4981 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-4274; PMID: 17510429
- Mitsiades N, Poulaki V, Mitsiades C, Tsokos M. Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res 2001; 61:2704 - 12; PMID: 11289151
- Seki N, Toh U, Sayers TJ, Fujii T, Miyagi M, Akagi Y, et al. Bortezomib sensitizes human esophageal squamous cell carcinoma cells to TRAIL-mediated apoptosis via activation of both extrinsic and intrinsic apoptosis pathways. Mol Cancer Ther 2010; 9:1842 - 51; http://dx.doi.org/10.1158/1535-7163.MCT-09-0918; PMID: 20515944
- Lu G, Punj V, Chaudhary PM. Proteasome inhibitor Bortezomib induces cell cycle arrest and apoptosis in cell lines derived from Ewing’s sarcoma family of tumors and synergizes with TRAIL. Cancer Biol Ther 2008; 7:603 - 8; http://dx.doi.org/10.4161/cbt.7.4.5564; PMID: 18223318
- Kontny HU, Hämmerle K, Klein R, Shayan P, Mackall CL, Niemeyer CM. Sensitivity of Ewing’s sarcoma to TRAIL-induced apoptosis. Cell Death Differ 2001; 8:506 - 14; http://dx.doi.org/10.1038/sj.cdd.4400836; PMID: 11423911
- Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 2009; 459:428 - 32; http://dx.doi.org/10.1038/nature08012; PMID: 19363473