Abstract
Background: Over-expression of epidermal growth factor receptor in esophageal cancer is associated with poor prognosis. The present study was conducted to evaluate safety and preliminary efficacy of nimotuzumab, a humanized anti-EGFR antibody in combination with radiation and chemotherapy in advanced esophageal tumours.
Patients and Methods: A Phase II clinical trial was conducted, where patients received cisplatin, 5-fluorouracil, and radiotherapy, either alone or combined with six weekly infusions of nimotuzumab at the dose of 200 mg. Safety was the primary endpoint. The antitumoral objective response rate was the secondary endpoint. Epidermal growth factor receptor expression, KRAS mutation status and anti-idiotypic response were also evaluated.
Results: Sixty-three patients were included in the study. Thirty patients were entered into the control group, and thirty-three patients received the treatment with nimotuzumab. The antibody was very well tolerated. Objective response rate was 47.8 % (nimotuzumab group) and 15.4 % (control group). Disease control rate was 60.9 % (nimotuzumab group) and 26.9 % (control group). Response and disease control rate were higher in patients with EGFR overexpressing tumors.
Conclusion: Nimotuzumab plus chemoradiotherapy was safe and provided statistically significant objective response. A Phase III in patients with similar characteristics will be launched.
Introduction
Cancer is at present a serious health problem. The number of cancer cases and deaths is projected to double up over the next 20–40 y. Cancer is now the third leading cause of death, with more than 12 million new cases and 7.6 million cancer deaths estimated to have occurred globally in 2007.Citation1
The incidence of esophageal cancer has increased in the last few decades together with changes in the type and location of the primary tumor.Citation2,Citation3 Radiation therapy administered concurrently with chemotherapy is the standard treatment in patients with non-resectable esophageal cancer. The most accepted chemotherapy treatment for this type of tumor is cisplatin combined with 5-fluorouracil (5-FU).Citation4-Citation6 The use of chemotherapy alone or chemotherapy plus radiation before surgery is the focus of several studies.Citation6-Citation10
Concurrent chemo-radiotherapy is a valuable treatment option for advanced esophageal cancer (EC), but improvement is still needed. Therefore, new therapies such as immunotherapy could become a new type of treatment for esophageal cancer.Citation11-Citation14 Epidermal growth factor receptor (EGFR) pathway has been implicated in pathophysiology of many epithelial malignancies, including esophageal cancer.Citation15-Citation17 Overexpression of EGFR in esophageal cancer is associated with poor prognosis.Citation17
Nimotuzumab is produced at the Center of Molecular Immunology (CIM) in Cuba. It is a humanized monoclonal antibody that recognizes an epitope located in the extracellular domain of the EGFR.Citation18 This monoclonal antibody is well tolerated and has proven efficacy in the treatment of advanced squamous cell carcinomas of the head and neck and high grade glioma patients.Citation19,Citation20 Nimotuzumab has demonstrated encouraging clinical results in the absence of severe side-effects observed with other approved anti-EGFR antibodies. Experimental observations demonstrated that in contrast to cetuximab, the intrinsic properties of nimotuzumab requires bivalent binding for stable attachment to the cellular surface, leading to nimotuzumab selectively binding to cells that have moderate to high EGFR expression levels.Citation21,Citation22
A phase II trial was conducted to study the safety and preliminary efficacy of nimotuzumab combined with radiation and chemotherapy in patients with non-resectable, epithelial origin esophageal cancer.
Results
Patient characteristics
Sixty-eight patients with non-resectable, epithelial origin esophageal cancer were included between December 2005 and December 2008. Five of these patients were randomized but did not receive any treatment, including chemo or radiotherapy. Thirty-three patients received nimotuzumab in combination with radiation and chemotherapy. The remaining 30 patients received radiation and chemotherapy alone. shows the principal demographic and tumor characteristics. In general, both arms were well balanced regarding the most important prognostic features. The majority of patients were male (80.65%) with a good ECOG performance status (0–1) (88.1%). Most patients were bearing stage III tumors (76.7%), which were located predominantly in the medium/thoracic esophagus (91.3%). One third of the patients (36%) had moderately differentiated tumors.
Table 1. Patients demographic and tumor characteristics
Toxicity
Treatment was well tolerated. The majority of the adverse events (in spite of the causal relationship) were classified as mild or moderate. Within each group, there were 23 and 25 (nimotuzumab vs. control arm) severe or very severe adverse events. Two toxic deaths were reported in the nimotuzumab arm, while four deaths were connected to adverse events in the control arm. None of the death was attributed to the investigational drug ().
Table 2. . Safety profile in a Phase II nimotuzumab clinical trial as CTC Version 3.0
All nimotuzumab related adverse events were categorized as grade 1 or 2 (nine) and consisted on fever (11.1%), headache (22.2%), high blood pleasure (11.1%), nausea(11.1%), phlebitis (22.2%), deglution pain (22.2%). None of the nimotuzumab treated patients had allergic reactions or skin rash. In summary, toxicity of chemoradiotherapy was not exacerbated by the addition of the humanized anti-EGFR mAb.
Efficacy
Antitumor response was analyzed in 49 patients. Patients were classified as evaluable (per protocol) if they received at least three doses of nimotuzumab, two cycles of chemotherapy and at least 40 Gy of cumulative radiation. Fourteen patients were considered non-evaluable on account of premature treatment discontinuation and the lack of confirmative imaging evaluations. In spite of the treatment interruption, the mean and median survival times of these 14 patients was equivalent to 10.02 and 8.87 mo, and were not classified as early progressors.
Per protocol, the objective response rate (complete response, CR + partial response, PR) was 47.8% for the nimotuzumab group and 15.4% for the control group (chi square p = 0.014). Disease Control Rate (DCR), (CR + PR + stable disease, SD) was 60.9% for nimotuzumab group and 26.9% for the control group (Chi square p = 0.017). Complete response was observed in six of the 23 evaluable patients treated with nimotuzumab and in three out of the 26 control patients.
Molecular Studies
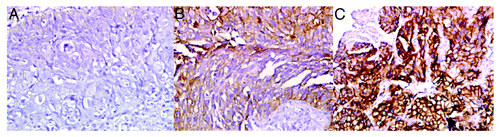
The EGFR expression at baseline was evaluated in 18 patients´ biopsies (13 from the nimotuzumab arm and five from the control arm). EGFR expression was classified as high in 10 of the 13 patients treated with nimotuzumab (77%) and in four out of the five control subjects (80%). For nimotuzumab treated patients that overexpress EGFR, objective response was 60% and DCR was 80%, which compares very favorably with the response rate seen in the intent to treat population. Representative EGFR tumor staining are shown in . KRAS evaluation did not detect any somatic mutation in codons 12 and 13 using allele-specific real-time polymerase chain reaction, precluding any correlative evaluation.
Figure 1. Baseline representative EGFR tumor staining, EGFR expression was classified as high in 14 of 18 evaluated patients. A negative expression of EGFR. positive anti EGFR staining 2+, c.3+. All of them at 40x
None of the evaluated patients developed anti-idiotypic response against the humanized antibody. Even though, overall survival was not a trial endpoint, we made a preliminary analysis including the 63 patients that entered the study. The median survival time was 8.1 mo for the nimotuzumab arm and 2.97 for the control patients.
Discussion
Cancer of the esophagus continues to be a threat to public health. The common practice is esophagectomy for surgically resectable tumors and radiochemotherapy for locally advanced, unresectable tumors.Citation4,Citation7
The EGFR is constitutively expressed in many normal epithelial tissues, including the skin and hair follicle. Overexpression of EGFR occurs in many types of human tumors, including esophageal (92%), head and neck (90%), colorectal (72%), prostate (65%), bladder (65%), ovarian (60%), cervical (60%), pancreatic (89%), renal cell (50%), and lung (50%) cancers. Expression of EGFR correlates with poor prognosis and advanced disease.Citation29
The addition of EGFR inhibitors to radiation therapy has a sound rationale, since studies have demonstrated that radiation therapy leads to EGFR activation and overexpression in tumor cells, resulting in cell proliferation and increased DNA repair capacity for the radiation caused damage. For this reason, blockade of EGFR activity may increase tumor sensitivity to radiation therapy.Citation30
Nimotuzumab is a humanized monoclonal antibody with intermediate affinity binding to the EGFR extracellular moiety, which inhibits the signaling pathway associated to the receptor.Citation21,Citation22 In the preclinical setting, treatment with nimotuzumab resulted in anti-proliferative effects by cellular inhibition of cell cycle, together with anti-angiogenic and pro-apoptotic activity.Citation31 Nimotuzumab has shown so far antitumor activity (combined with radiation or chemo-radiation) in head and neck and high grade glioma patients.Citation19,Citation20 In addition, it is currently been tested in 25 clinical trials for other tumor localizations.
This clinical trial was designed to assess the safety and preliminary efficacy of combining nimotuzumab and chemoradiotherapy in unresectable esophageal cancer patients.
The antibody in combination with radiochemotherapy was very well tolerated. No serious adverse events were attributed to nimotuzumab and the antibody did not increase the toxicity of chemo-radiotherapy. Serious adverse events have been reported for other widely used anti-EGFR antibodies, already in the market, like cetuximab and panitumumab. Cetuximab can induce severe infusion reactions in approximately 3% of patients as well as cardiopulmonary arrest and sudden death in up to 2% of the patients. Panitumumab engenders dermatologic toxicities in 89% of patients (12% severe) and severe infusion reactions in approximately 1% of patients. Nimotuzumab does not induce acneiform rash or allergic reactions. No anti-idiotypic response was detected after 6 weekly doses, confirming the low antigenicity of the humanized antibody.
The trial was designed to demonstrate the safety of the triple agents’ combination. Treatment compliance was not high since only 49 patients received at least 40 Gy and 2 chemotherapy cycles. Gastrostomy or jejunostomy were not mandatory before inclusion and not all patients received nutritional support. The efficiency as a secondary endpoint in the study was also effective. Still, patients receiving immuno-radiochemotherapy had a significantly higher response rate and disease control rate. Moreover, patients with EGFR overexpressing tumors derived the greatest benefit in terms of tumor shrinkage or control. This result should be interpreted with caution since only a small subset of the biopsies was analyzed. Previous findings support the validity of EGFR expression as a predictive biomarker of nimotuzumab efficacy in head and neck and gastric cancer patients.Citation32-Citation34 Receptor expression has not been validated before as predictor for other EGFR antagonists. Recently, new data from the FLEX study showed that among lung cancer patients with high EGFR expression, the response rate was significantly increased by the addition of cetuximab to standard chemotherapy. No KRAS codon 12/13 tumor mutations were identified in the evaluated samples. A similar finding was published recently by a Lorenzen and coworkers,Citation35 meaning that KRAS mutation might not be frequent in esophageal tumors.
In the current trial, patients received up to 6 doses of nimotuzumb. Current clinical practice is to maintain the antibody treatment until progressive disease of unmanageable toxicity. The excellent safety profile of nimotuzumab has allowed its long-term use, even in pediatric population. More than 450 patients have received bi-weekly treatment for one year or more, and 135 for more than two years. There is no cumulative toxicity since the frequency of adverse events is the same after the first dose and after two years of treatment.Citation32
Limitations of this phase two studies include the small sample size, which limits our assessment of overall survival; potential investigator bias in assessing the rate of clinical benefit and progression-free survival but a Phase III in patients with similar characteristics will be launched.
In conclusion, nimotuzumab in combination with radiotherapy and cisplatin/5Fu-radiotherapy was well tolerated and significantly improves response rate. A Phase III trial, in the same patient population and with long-term use of nimotuzumab, will be launched, to evaluate if objective response definitely translate into survival benefit.
Methods
Patients
This was a multicenter and open-labeled Phase II study, where patients were randomized to receive radio-chemotherapy or radio-chemotherapy plus nimotuzumab. Patients were recruited at 5 hospitals in Cuba. The study was conducted under the principles described in the Declaration of Helsinki and according the International Conference of Harmonization´ Good Clinical Practices (ICH-GCP).Citation23 The protocol was approved by the National Regulatory Agency (CECMED) and the ethic committees of the participating hospitals. The protocol information was included on the National Register for clinical trials http://registroclinico.sld.cu/trials/clinical_trial_who.2010-10-26.7354026097, PCEC Unique ID number: RPCEC00000014, which is a primary register approved by the World Health Organization (WHO).
Selection criteria included patients with non-resectable, epithelial origin esophageal cancer in stage III or IV, located in cervical and intra thoracic (upper and mid-thoracic part) esophagus; patients who had never received treatment and who were candidates for radiation and chemotherapy at the time of inclusion; patients with measurable lesions (at least one dimension) and with a larger diameter greater than or equal to 20 mm using conventional measurements such as CT (CAT scan), radiography and ecography, or a larger diameter greater than or equal to 10 mm using Helicoidally Tomography Scan; age more than 18 and less than 75 years; Eastern Cooperative Oncology Group performance status (ECOG) ≤ 224 Karnofsky Performance Status (KPS) ≥ 60%Citation25; life expectancy greater than six months; normal organ and bone marrow function according to laboratory parameters; and female patients of child-bearing age with negative pregnancy test. Gastrostomy or jejunostomy before radiation and chemotherapy was not a selection criterion. The most important exclusion criteria consisted of patients with esophageal cancer located in the lower thoracic esophageal part, patients with allergy history to any of the components of nimotuzumab or to the chemotherapeutic agents used in the study, and patients with concurrent non-controlled diseases such as symptomatic congestive heart failure and cardiac arrhythmia. Criteria for discontinuing treatment with radiation and chemotherapy plus nimotuzumab included: progression of disease; grade III or IV adverse events (AE); and interrupting the radiation therapy more than three times for periods longer than seven days, or more than twice for periods longer than 10 days, or for a period longer than 15 days (at once).
Treatment
Patients were randomized in two groups to receive radiation and chemotherapy alone or in combination with nimotuzumab. Radiation and chemotherapy were administered as follows: the planned overall treatment time was five weeks, ionizing radiation via megavoltage (60 Cobalt) were delivered in doses of 180–200 cGy given once daily, five days per week to a total dose of 4500 to 5000 cGy, via lateral opposed portals encompassing the primary tumor. For planning, each patient underwent CT (CT) scan; simulation with radiographic control was performed.
Chemotherapy consisted on Cisplatin 75mg/m2, four cycles on the second day of the 2th, 6th, 10th and 14th weeks of treatment and 5-Fluorouracil (5-FU) 750 mg/m2, four cycles, continuous infusion, from days two to five, of the 2th, 6th, 10th and 14th weeks of treatment. Six doses of nimotuzumab (200 mg fixed doses) were administered to patients randomized into the treatment arm. The antibody was administered by intravenous injection (antecubital vein), in 250 ml of saline solution.
Patient evaluation
Physical exam of the patient, laboratory tests and imageneologic evaluations were carried before randomization. Adverse events´ severity was graded according to National Cancer Institute’s Common Toxicity Criteria (NCI-CTC), version 3.0. In addition, adverse events were classified according the causal relationship, seriousness, the outcome and expectiveness.Citation24 The anti-tumoral effect of treatment was evaluated according to RECIST guidelines.Citation26 The base value was taken as the measurement made 4 weeks before starting treatment. Tumoral lesions were also measured one week after radiation and chemotherapy were completed (18 weeks after initiated the treatment) as established in the protocol, and every 3 mo. The evaluation of the patients was performed through CT (CT scan) in all cases
For a subset of patients, baseline tumor biopsies were analyzed in order to evaluate the correlation between EGFR expression, K-RAS mutation status and objective response rate. A commercially available kit from DAKO (EGFR pharm-DxTM Kit) was used for EGFR expression evaluation in 18 patients (13 from the nimotuzumab arm and five from control arm). Briefly, following incubation with the primary monoclonal antibody, clone 2–18C9, to human EGFR protein, the reaction was revealed with a secondary goat anti-mouse antibody and horseradish peroxidase linked to a dextran polymer backbone. Control slides containing 2 formalin-fixed, paraffin-embedded human cell lines with staining intensity scores of 2+ and 0 were used for quality control. Digitalizing of histological cut images was performed through a microscope coupled to a system of digital video cameras with an image analyzer system.Citation27
The same tumor biopsies were used for KRAS analysis, as follows: formalin-fixed, paraffin-embedded tumor sections were deparaffinized and air-dried. DNA was isolated using proteinase K and a DNeasy minispin column (Qiagen, Valencia, CA). Mutant KRAS was detected using a validated KRAS mutation kit (DxS Ltd, Manchester, United Kingdom) that identifies seven somatic mutations located in codons 12 and 13 using allele-specific real-time polymerase chain reaction (RT-PCR) established acceptance criteria. The KRAS analysis was performed in a blinded fashion and appropriate quality controls for each assay were included (Collaboration with Diagnomol SA laboratory, Mexico DF)Citation28
In 15 patients, anti-idiotypic response against nimotuzumab was evaluated. Samples for the anti-idiotypic response were taken every 2 weeks within the first 6 treatment weeks, and later on, monthly until one year. The anti-idiotypic response against nimotuzumab was measured by a previously described method.Citation19,Citation20 Pre-treatment serum samples of each patient were used as controls. The assay was considered positive when post-treatment/pre-treatment ratio was higher than two.
Statistical analysis
The primary endpoint of the trial was safety of combining nimotuzumab with chemotherapy and radiotherapy. The objective response to treatment was a secondary endpoint in the study. Chi Square and Fisher exact tests were used to evaluate the homogeneity of the groups for qualitative variables, and to compare the antitumor response between treatment groups. Epidermal growth factor receptor (EGFR) expression was correlated with response rate according by Chi Square and Fisher exact tests.
Author’s Note:
Please cite references Citation36-Citation42
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
REFERENCES
- Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis 2010; 31:100 - 10; http://dx.doi.org/10.1093/carcin/bgp263; PMID: 19934210
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr.. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991; 265:1287 - 9; http://dx.doi.org/10.1001/jama.1991.03460100089030; PMID: 1995976
- Devesa SS, Blot WJ, Fraumeni JF Jr.. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83:2049 - 53; http://dx.doi.org/10.1002/(SICI)1097-0142(19981115)83:10<2049::AID-CNCR1>3.0.CO;2-2; PMID: 9827707
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer. Long-term follow-up of prospective randomized trial (Radiation Therapy Oncology Group 85-01). JAMA 1999; 281:1623 - 7; http://dx.doi.org/10.1001/jama.281.17.1623; PMID: 10235156
- Nemoto K. Current Status of Radiation Therapy-Evidence-based Medicine (EBM) of addition Therapy-Current Management of Patients with Esophageal Cancer. Nippon Igaku Hoshasen Gakkai Zasshi 2001; 62:132 - 7
- Chong G, Cunningham D. Gastrointestinal cancer: recent developments in medical oncology. Eur J Surg Oncol 2005; 31:453 - 60; http://dx.doi.org/10.1016/j.ejso.2005.02.026; PMID: 15922879
- Bates BA, Detterbeck FC, Bernard SA, Qaqish BF, Tepper JE. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol 1996; 14:156 - 63; PMID: 8558191
- Arnott SJ, Duncan W, Gignoux M, Hansen HS, Launois B, Nygaard K, et al. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev 2005; CD001799; PMID: 16235286
- Ku GY, Ilson DH. Esophageal cancer: adjuvant therapy. Cancer J 2007; 13:162 - 7; http://dx.doi.org/10.1097/PPO.0b013e318074dbe7; PMID: 17620765
- Kersting S, Konopke R, Dittert D, Distler M, Rückert F, Gastmeier J, et al. Who profits from neoadjuvant radiochemotherapy for locally advanced esophageal carcinoma?. J Gastroenterol Hepatol 2009; 24:886 - 95; http://dx.doi.org/10.1111/j.1440-1746.2008.05732.x; PMID: 19655439
- Czito BG, Willett CG. In Pursuit of Progress: Multimodality Strategies Will Form the Cornerstone of Cure for Esophageal Cancer. Gastrointest Cancer Res 2009 Mar–Apr; 3(2): 74–76.
- Lagarde SM, Vrouenraets BC, Stassen LP, van Lanschot JJ. Evidence-based surgical treatment of esophageal cancer: overview of high-quality studies. Ann Thorac Surg 2010; 89:1319 - 26; http://dx.doi.org/10.1016/j.athoracsur.2009.09.062; PMID: 20338377
- Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol 1989; 142:3714 - 25; PMID: 2785562
- Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Arch Surg 1992; 127:1446 - 50; http://dx.doi.org/10.1001/archsurg.1992.01420120080015; PMID: 1365692
- Philip PA, Ajani JA. Has combined modality therapy improved the outlook in carcinoma of the esophagus?. Oncology (Williston Park) 1994; 8:37 - 42, discussion 44-5, 50-2, 61; PMID: 7527642
- Toh U, Yamana H, Sueyoshi S, Tanaka T, Niiya F, Katagiri K, et al. Locoregional cellular immunotherapy for patients with advanced esophageal cancer. Clin Cancer Res 2000; 6:4663 - 73; PMID: 11156218
- Ekman S, Bergqvist M, Tell R, Bergström S, Lennartsson J. Hsp90 as a therapeutic target in patients with oesophageal carcinoma. Expert Opin Ther Targets 2010; 14:317 - 28; http://dx.doi.org/10.1517/14728221003621278; PMID: 20148718
- Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res 2002; 22:1737 - 54; PMID: 12168863
- Solit DB, Rosen N. Hsp90: a novel target for cancer therapy. Curr Top Med Chem 2006; 6:1205 - 14; http://dx.doi.org/10.2174/156802606777812068; PMID: 16842157
- Giezen TJ, Mantel-Teeuwisse AK, Straus SM, Schellekens H, Leufkens HG, Egberts AC. Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA 2008; 300:1887 - 96; http://dx.doi.org/10.1001/jama.300.16.1887; PMID: 18940975
- Deonarain MP, Kousparou CA, Epenetos AA. Antibodies targeting cancer stem cells: a new paradigm in immunotherapy?. MAbs 2009; 1:12 - 25; http://dx.doi.org/10.4161/mabs.1.1.7347; PMID: 20046569
- Dragovich T, Campen C. Anti-EGFR-Targeted Therapy for Esophageal and Gastric Cancers: An Evolving Concept. J Oncol 2009:804108. Available in: http://www.ncbi.nlm.nih.gov/pubmed/19636422.
- Mateo C, Moreno E, Amour K, Lombardero J, Harris W, Pérez R. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology 1997; 3:71 - 81; http://dx.doi.org/10.1016/S1380-2933(97)00065-1; PMID: 9154469
- Crombet TC, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 2004; 22:1646 - 54; http://dx.doi.org/10.1200/JCO.2004.03.089; PMID: 15117987
- Ramos TC, Figueredo J, Catala M, González S, Selva JC, Cruz TM, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther 2006; 5:375 - 9; http://dx.doi.org/10.4161/cbt.5.4.2522; PMID: 16575203
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649 - 55; http://dx.doi.org/10.1097/00000421-198212000-00014; PMID: 7165009
- Karnofsky D, Abelmann W, Craver L, Burchenal J. The use of nitrogen mustard in the palliative treatment of cancer. Cancer 1948; 1:634 - 56; http://dx.doi.org/10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205 - 16; http://dx.doi.org/10.1093/jnci/92.3.205; PMID: 10655437
- European organization for research and treatment of cancer.EORTC QLQ-C30. Available from: http://groups.eortc.be/qol/downloads/modules/specimen_20qlq_c30.pdf
- European organisation for research and treatment of cáncer. EORTC QLQ – OES18. Available from: http://groups.eortc.be/qol/downloads/modules/specimen_20qlq_oes18.pdf
- Essack M, Radovanovic A, Schaefer U, Schmeier S, Seshadri SV, Christoffels A, et al. DDEC: Dragon database of genes implicated in esophageal cancer. BMC Cancer 2009; 9:219; http://dx.doi.org/10.1186/1471-2407-9-219; PMID: 19580656
- Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol 2009; 27:1130 - 6; http://dx.doi.org/10.1200/JCO.2008.19.8168; PMID: 19124802
- Torres OJ, Peña L, Martínez LB, Padilla D, Rodríguez K. Impact of the GCP System in the Clinical Trials. Control Clin Trials 2003; 24:126S
- Ilson DH. New developments in the treatment of esophageal cancer. Clin Adv Hematol Oncol 2004; 2:97 - 104; PMID: 16163169
- Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, et al. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res 2006; 12:6064 - 72; http://dx.doi.org/10.1158/1078-0432.CCR-06-0910; PMID: 17062682
- Jiménez-Medina E, Berruguilla E, Romero I, Algarra I, Collado A, Garrido F, et al. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 2008; 8:78; http://dx.doi.org/10.1186/1471-2407-8-78; PMID: 18366723
- Liu W, Zhang X, Sun W. Developments in treatment of esophageal/gastric cancer. Curr Treat Options Oncol 2008; 9:375 - 87; http://dx.doi.org/10.1007/s11864-009-0097-1; PMID: 19396633
- Wang W, Xue L, Liu H, Wang P, Xu P, Cai Y. Aberrant changes of Wnt2/beta-catenin signaling pathway induced by sodium nitroprusside in human esophageal squamous cell carcinoma cell lines. Cancer Invest 2010; 28:230 - 41; http://dx.doi.org/10.3109/07357900903095698; PMID: 19857041
- Wang ZG, Fukazawa T, Nishikawa T, Watanabe N, Sakurama K, Motoki T, et al. RAD001 offers a therapeutic intervention through inhibition of mTOR as a potential strategy for esophageal cancer. Oncol Rep 2010; 23:1167 - 72; PMID: 20204306
- Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Azuma M, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res 2009; 3:153 - 61; PMID: 19742141
- Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009; 20:1667 - 73; http://dx.doi.org/10.1093/annonc/mdp069; PMID: 19549707
- National Comprehensive Cancer Network. NCCN Guidelines and Clinical Resources. Access 2011 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp