Abstract
Anaplastic thyroid carcinoma (ATC) is an extremely aggressive and rapidly fatal neoplasm. The aim of this study was to identify a limited cell cycle associated protein expression pattern unique to ATC and to correlate that pattern with clinical outcome. This represents one of the largest tissue micro-array projects comparing the cell cycle protein expression data of ATC to other well-differentiated tumors in the literature. Tissue microarrays were created from 21 patients with ATC and an age and gender matched cohort of patients with papillary thyroid carcinoma (PTC). Expression of epidermal growth factor receptor, cyclin D1, cyclin E, p53, p21, p16, aurora kinase A, opioid growth factor (OGF), OGF-receptor, thyroglobulin and Ki-67 was evaluated in a semi-quantitative fashion. Differences in protein expression between the cohorts were evaluated using chi-square tests with Bonferroni adjustments. Survival time and presence of metastasis at presentation were collected. The ATC cohort showed a statistically significant decrease (p < 0.05) in thyroglobulin expression and statistically significant increases (p < 0.05) in Ki-67 and p53 expression as compared with the PTC cohort. A trend toward loss of p16 and p21 expression was noted in the ATC cohort. A trend toward decreased survival was noted with p21 expression. These data indicate disruption of the normal cell cycle with aberrant expression of multiple protein markers suggesting increased proliferative activity and loss of control of cell cycle progression to G1 phase. These findings support the assertion that ATC may represent the furthest end of a continuum of thyroid carcinoma dedifferentiation.
Introduction
Anaplastic thyroid carcinoma (ATC) is an aggressive and rapidly fatal endocrine neoplasm. Even with multimodality therapy including surgical excision, fractionated radiotherapy and chemotherapy; death commonly occurs within six months for most patients.Citation1-Citation4 As with many other human cancers, investigations to identify and characterize specific alterations of the cell cycle may lead to a better understanding of the biology of this disease. Given the ubiquitous nature of p53 mutations in most other human carcinomas, mutations in p53 have been the most consistently identified mutation in ATC within the literature, with reports of approximately 30% to nearly 70% of ATC harboring this mutation.Citation5-Citation7 Multiple studies have reported overexpression of either mRNA and/or protein for β-catenin,Citation3 cyclins D1 and E,Citation3,Citation6,Citation8 and epidermal growth factor receptor (EGFR).Citation3,Citation9-Citation11 In fact, nearly 80% of anaplastic thyroid cancers evaluated in one study had EGFR abnormalities.Citation9 All of the aforementioned proteins are important for progression through the cell cycle. Regarding cell cycle mediators responsible for inhibition of the cell cycle such as p16 and p21, even fewer studies have been performed and have conflicting results in whether these markers are overexpressed or under-expressed in ATC.Citation7,Citation8
It is unclear whether derangements in these cell cycle mediators represent unique changes present within ATC or if they are part of a spectrum of molecular disturbances that occur in thyroid carcinomas in general. Several studies have shown progression in aberrant molecular and protein expression such as p53 and Ki-67 along the spectrum of well to intermediate to poorly differentiated thyroid tumors.Citation5,Citation7,Citation12-Citation14 Furthermore a history of a prior well-differentiated thyroid neoplasm is present in up to 50% of patients with anaplastic thyroid tumorsCitation1 and the literature reports a wide range, 25 to 90%, of patients have a concurrently diagnosed well differentiated thyroid carcinoma at presentation.Citation1,Citation6,Citation14 This begs the question whether all of ATC arise from prior well differentiated thyroid carcinomas or if the presence of an ATC can actually imply a de novo tumor.
Given its accelerated clinical course and uncommon occurrence, there is much that still needs to be studied regarding derangements in cell cycle mediators of ATC. Thus, the aim of this study was to identify a limited cell cycle associated protein expression pattern unique to ATC that was not present in well-differentiated thyroid carcinoma. We evaluated thyroglobulin and KI-67 protein expression as markers for comparison with prior ATC studies. EGFR, cyclin D1 and cyclin E were used to evaluate potential derangements in cell cycle mediators that promote progression of G1 phase to S phase in normal cells. Aurora kinase A was used to evaluate potential derangements in mitosis. Opioid growth factor (OGF), OGF receptor (OGFr), p53, p21 and p16 were used to evaluate potential aberrant expression of cell cycle proteins that inhibit progression of G1 phase to S phase in normal cells. To our knowledge, this study represents one of the largest tissue micro-array projects comparing the cell cycle mediators protein expression data of ATC to other well-differentiated tumors in the literature.
Results
The median age of each cohort was 67-y-old with an equal gender distribution. For those patients with ATC, the median survival time was four months with four patients surviving greater than one year. By convention, ATC is considered stage IV disease. Seventeen of the patients in this study presented at stage IV-C. All of the patients whom survived greater than one year had stage IV-A or IV-B disease. Fifteen of the patients with ATC had radiologically and/or pathologically confirmed metastasis at the time of diagnosis. Three of the patients had no metastasis documented and each of these patients had stage IV-A or IV-B disease. For the remaining three patients, information regarding metastatic disease was not available. Surgical resection of the ATC was performed in 11 patients.
lists the protein expression data for both cohorts. provides H&E examples of the tissue cores used for the ATC cohort and PTC cohort, respectively. Thyroglobulin expression was lost in 78.9% of the ATC cohort as compared with 4.8% of the PTC cohort. The expression of p53 () and Ki-67 in the ATC cohort was 52.6% and 85%, respectively. In comparison, p53 () and Ki-67 expression was present in 0% and 14.3% of PTC cohort cases, respectively. Expression of cyclin D1 (75% of the ATC cohort, 90.5% of the PTC cohort) (, respectively), cyclin E (35% of the ATC cohort, 9.5% of the PTC cohort), aurora A (90% of the ATC cohort, 100% of the PTC cohort), EGFR (100% of the ATC cohort, 95.2% of the PTC), OGF (95% of the ATC cohort, 100% of the PTC cohort), and OGFR (89.5% of the ATC cohort, 61.9% of the ATC cohort) was similar between both cohorts. The expression of p16 was identified in 31.6% of cases within the ATC cohort and 66.7% of cases within the PTC cohort. The expression of p21 was present in 30% of cases within the ATC cohort and 66.7% of cases within the PTC cohort (, respectively). Ultimately, the ATC cohort showed a statistically significant decrease in thyroglobulin expression (p < 0.05) and statistically significant increases in Ki-67 (p < 0.05) and p53 (p < 0.05) expression as compared with the PTC cohort.
Table 1. Anaplastic vs. papillary thyroid carcinoma: Immunohistochemical results
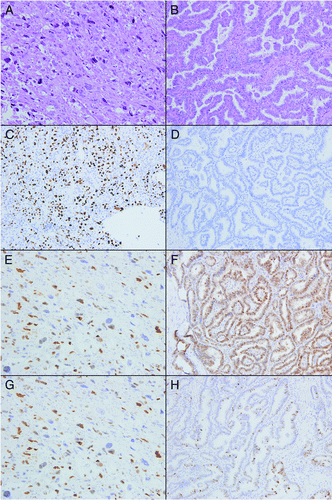
Figure 1. Hematoxylin and eosin stained sections of anaplastic (A) and papillary (B) thyroid carcinoma. ATC (C) showed a significant increase in staining for p53 as compared with PTC (D). Both ATC (E) and PTC (F) showed diffuse positive staining for cyclin D1. Although not statistically significant, ATC (G) was less likely to be p21 positive than PTC (H). Original magnification 200 x for all images.
No significant associations between protein marker expression and survival or presence of metastasis were noted. However, a trend toward decreased survival was noted in ATC expressing p21.
Discussion
ATC is an aggressive disease with a nearly uniformly fatal outcome. The data from this study indicate a disruption of the normal cell cycle with aberrant expression of multiple protein markers in ATC. Thyroglobluin, p53 and Ki-67 results were consistent with the findings of prior studies that show overexpression of Ki-67 and p53 and underexpression of thyroglobulin in ATC.Citation5-Citation7,Citation12-Citation17 As an important tumor suppressor gene, p53 is found to be mutated in many other human malignant tumors. It serves as both an inhibitor of G1 phase as well as a pro-apoptotic molecular when the proper cellular conditions are met. Our results confirm findings discovered in several prior studies that show increased aberrant p53 expression is seen as tumor differentiation is lost. This suggests that p53 mutations may be an important late factor in tumorigenesis of ATC.Citation5,Citation6,Citation13 The p53 mutations are likely a late but critical event in this pathway resulting in the deregulation of an important tumor suppressor protein which may in part explain the rapid clinical decline seen in ATC. Ki-67 serves as a marker of cellular proliferation which is present in the active phases of the cell cycle. Its increased production correlates well with histologic criteria of proliferation including numerous mitoses.
A trend toward the loss of expression of p21 was seen in the ATC cohort as compared with the PTC cohort. This was, however, not statistically significant. Our results are consistent with prior studies showing some, albeit decreased, p21 expression in ATC.Citation3 Loss of p21 expression has been more pronounced in other studies including one in which of 22 insular and 38 ATC, p21 was expressed in only 3% of the ATC and none of the insular carcinomas.Citation5 Similarly, another study revealed a lack of expression of p21 in ATC as compared with poorly differentiated and well differentiated thyroid tumors.Citation7 This trend inversely correlates the increase in p53 expression seen in the ATC cohort. This finding suggests that the loss of p21 expression in ATC is secondary to decreased p21 activation caused by the production of an altered p53. Interestingly, although not statistically significant, patients without p21 expression in our study showed a trend toward increased 12 mo survival as compared with those with p21 expression. A larger study sample is needed to determine the true significance of these results.
We found that cyclin D1 and cyclin E expression patterns were similar in both cohorts indicating that loss of cell cycle control over the G1 checkpoint is a common event in both tumors. Cyclin D1 is important to the early checkpoint and cyclin E is important to the late checkpoint in this phase of the cell cycle. It might be expected to see an increase in cyclin D1 expression; either primary to a specific mutation targeting upregulation of this gene or secondary to increased expression of an upstream proliferative marker. Our data showed a trend toward loss of cyclin D1 expression in the ATC cohort as compared with the PTC cohort although most specimens were cyclin D1 positive. A prior study of 21 ATC demonstrated no staining of cyclin D1Citation7 whereas other studies have shown much higher levels of cyclin D1 expression in ATCCitation3 as well as positive expression in other malignant thyroid tumors.Citation8 Cyclin E expression has also been shown in various thyroid tumors including ATCCitation3,Citation14 as well as well differentiated tumors.Citation8 We theorize that the conflicting results in multiple studies regarding the cyclins represent the ability of this aggressive tumor to acquire additional genetic mutations that are not completely necessary for tumorogenesis and thus are not a consistent aberration seen in all ATC. An alternate explanation is that direct alterations in cyclin D1 protein structure can occur in ATC making a specific antibody clone less likely to show immunoreactivity. Another hypothesis includes that cyclin D1 expression may be truly downregulated as this tumor has found other avenues to induce proliferation. Further study, including looking at DNA status of the cyclin genes in ATC is warranted.
EGF binds its receptor on the cell membrane and serves to induce cyclin D1 binding of CDK4. In prior studies, approximately 25% to 95% of studied ATC cases showed EGFR overexpression via immunohistochemistry.Citation3,Citation9,Citation14,Citation18,Citation19 Our data supports the theory that EGFR expression is upregulated early in the carcinoma sequence of the thyroid as most of the PTC and all of the ATC cohort demonstrated expression of EGFR. As in other studies, EGFR expression seems to be present in well differentiated and undifferentiated tumors of the thyroid.Citation14,Citation19 Interestingly despite the presence of increased protein expression, coding mutations are rarely seen in these tumors. Lee et al.l demonstrated that even in non-EGFR protein expressing tumors, the EGF gene seemed to be polysomic.Citation20 These changes may indicate an early event in thyroid tumorogenesis.
A major inhibitor of the cyclinD1/CDK4 complex in the early G1/S checkpoint is p16. In our study, a trend toward loss of p16 expression was noted in the ATC cohort. This suggests loss of an inhibitory mediator of the G1 checkpoint. Interestingly, a prior study evaluating ATC cases with concurrent differentiated thyroid carcinomas showed an increase in p16 expression in ATC in approximately 25% of cases reviewed.Citation14 Another study of well-differentiated tumors and benign thyroid conditions showed relatively more p16 staining in the tumor groups as compared with the benign conditions.Citation8 Several possibilities may explain these conflicting results. Perhaps p16 expression in well differentiated tumors reflects relatively normal control of this checkpoint. With an undifferentiated tumor, either p16 no longer plays a role in tumorogenesis or as additional mutations within cell cycle pathway occur this may lead to a loss of expression of this protein.
The aurora kinase proteins are important to effective mitosis and cytokinesis. Mutations of any of these proteins can lead to an unstable chromosomal content of the cell. Aberrant expression of aurora A and C has been identified in ATC.Citation3,Citation21,Citation22 Our results confirm these prior findings but also show that aurora kinase A expression is found in the vast majority of PTC as well. This may indicate that aurora kinase A expression is an early event in the evolution of most thyroid carcinomas.
Although this study represents one of the largest cohorts of ATC studied, it is still limited by its sample size. This study employed more stringent cut-offs for interpreting whether or not an immunohistochemical stain was positive than most other studies. A lower threshold for calling the immunohistochemical stains positive may have shown more drastic differences between the PTC and ATC cohorts.
In conclusion, this study represents one of the largest comparisons in cell cycle mediator protein expression patterns between ATC and well-differentiated carcinoma in the literature. We found that ATC is associated with significant changes in expression of Ki-67, p53 and thyroglobulin as compared with PTC. These findings lend support to the assertion that ATC may represent the furthest end of a continuum of thyroid carcinoma dedifferentiation. Furthermore, reduced expression of p21 and p16 indicate that there are multiple derangements in the progression of the normal cell cycle with loss of tumor suppressor gene effects. EGFR, OGF and OGFR expression is present in nearly all cases within the ATC cohort, however the significance of expression of these proteins in the pathogenesis of ATC remains unknown. Further characterization of the protein expression profile of ATC, including evaluation of p21 expression correlations with clinical factors in a larger cohort, may allow for better diagnostic as well as potential targeted chemotherapeutic strategies for these patients.
Materials and Methods
Forty cases of ATC between 1977 and 2009 were identified using a tumor registry at Penn State Milton S Hershey Medical Center. Of these cases, slides from 28 unique patients were obtained and the histological diagnosis of ATC was confirmed by two pathologists. Paraffin embedded tissue was available for 21 of these patients and used to construct a tissue microarray consisting of two cores of each specimen, each 2 mm in diameter, on a Beecher MTA-I.
Corresponding age and gender matched controls (± 5 y) of patients diagnosed with papillary thyroid carcinoma (PTC) were also identified through a tumor registry and the diagnosis was confirmed by two pathologists. Similarly, two cores of tumor, each 2 mm in diameter, were retrieved from each case and used to create a separate tissue microarray. Five um sections were created from each microarray, deparaffinized and rehydrated for preparation for hematoxylin/eosin and immunohistochemical (IHC) staining.
Commercially available immunohistochemical stains were obtained for the following proteins: thyroglobulin (clone DAK-Tg6 from Dako at dilution 1:800, EDTA), p53 (clone 318–6-11 from Dako at dilution 1:200, EDTA), KI-67 (clone MIB-1 from Dako at dilution 1:100, EDTA), EGFR (clone H11 from Dako at dilution 1:100, proteolytic enzymes), cyclin D1 (clone SC-718 from Santa Cruz Biotechnology at 1:200, EDTA), cyclin E (clone 13A3 from Lab Vision at dilution 1:100, EDTA), aurora A (clone N-20 from Santa Cruz Biotechnology at dilution 1:100, EDTA), p21 (clone SX118 from Dako at dilution 1:100, EDTA), and p16 (clone E6H4 from Ventana at dilution 1:25, EDTA) . Positive controls were used for each antibody.
IHC was performed by first using heat-induced epitope retrieval in 10 mM citrate buffer pH 6.0 or 1 mM EDTA pH 8.0 for 20 min with 20 min of cooling at room temperature. This was followed by a 10 min 3% peroxidase block at room temperature. Incubation with the primary antibodies was then performed at room temperature. This was followed by incubation with Envision+ä (labeled polymer) goat anti-mouse or rabbit/HRP (Dako Cytomation) for 30 min at room temperature. Staining was completed with chromagen development of DAB+ substrate (Dako Cytomation) for 10 min followed by counterstaining for 6 min in Mayer’s modified hematoxylin (Dako Cytomation).
OGF and OGFr immunohistochemistry was performed by incubating slides with the appropriate antibody diluted in 1:250 Sorenson’s phosphate buffer. After a wash step, the slides were incubated with a secondary antibody, goat anti-rabbit IgG, conjugated to rhodamine. Tissue sections incubated with the secondary antibody only were used as a control. Slides were viewed using fluorescence microscopy.
Each immunohistochemical stain was analyzed by two pathologists independently and semi-quantitatively assigned a score 0–5 based on the percentile range of tumor staining (0 = 0–5%; 1 = 6–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–95%; 5 = 96–100%). Scores were averaged across the pathologists and the average score was dichotomized as ≤ 25% (considered negative) vs. > 25% (considered positive), to form binary expression data. Clinical data including demographic information, survival time (time from diagnosis to death or last known update), extent of tumor, stage of disease, presence of metastases and treatment was obtained to allow for characterization of the clinical course of ATC.
Differences in protein expression between the anaplastic and papillary carcinoma groups were tested using chi-square tests with Bonferroni adjustments applied to p-values. Within the anaplastic carcinoma group, survival and metastasis were evaluated by protein expression (≤ 25% vs. > 25%). For survival, Kaplan-Meier curves were constructed for stains with ≥ 5 subjects in each group and the logrank test was used to compare survival between groups. Fisher’s Exact Test was used to evaluate metastasis by expression of immunohistochemical stain.
Disclosure of Potential Conflicts of Interests
There are no financial interests related to this manuscript to disclose from any of the authors.
Acknowledgments
The authors are grateful to Patricia McLaughlin, PhD for her help with the OGF/OGFR staining as well as Eric Schaeffer for his help in statistical design of this project. This project was funded by a Penn State Milton S Hershey Medical Center Department of Pathology Research Initiation Grant.
References
- Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 2006; 13:453 - 64; http://dx.doi.org/10.1245/ASO.2006.05.042; PMID: 16474910
- Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am 2008; 37:525 - 38, xi; http://dx.doi.org/10.1016/j.ecl.2008.02.003; PMID: 18502341
- Wiseman SM, Masoudi H, Niblock P, Turbin D, Rajput A, Hay J, et al. Anaplastic thyroid carcinoma: expression profile of targets for therapy offers new insights for disease treatment. Ann Surg Oncol 2007; 14:719 - 29; http://dx.doi.org/10.1245/s10434-006-9178-6; PMID: 17115102
- Mitzer R, Goldenberg D, Durvesh S, Evans J. Anaplastic thyroid cancer: A three decade experience. [Abstract] Otolaryngol Head Neck Surg 2009; 141:144; http://dx.doi.org/10.1016/j.otohns.2009.06.459; PMID: 19559976
- Lam KY, Lo CY, Chan KW, Wan KY. Insular and anaplastic carcinoma of the thyroid: a 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg 2000; 231:329 - 38; http://dx.doi.org/10.1097/00000658-200003000-00005; PMID: 10714625
- Wiseman SM, Loree TR, Rigual NR, Hicks WL Jr., Douglas WG, Anderson GR, et al. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck 2003; 25:662 - 70; http://dx.doi.org/10.1002/hed.10277; PMID: 12884350
- Saltman B, Singh B, Hedvat CV, Wreesmann VB, Ghossein R. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery 2006; 140:899 - 905, discussion 905-6; http://dx.doi.org/10.1016/j.surg.2006.07.027; PMID: 17188136
- Melck A, Masoudi H, Griffith OL, Rajput A, Wilkins G, Bugis S, et al. Cell cycle regulators show diagnostic and prognostic utility for differentiated thyroid cancer. Ann Surg Oncol 2007; 14:3403 - 11; http://dx.doi.org/10.1245/s10434-007-9572-8; PMID: 17882495
- Ensinger C, Spizzo G, Moser P, Tschoerner I, Prommegger R, Gabriel M, et al. Epidermal growth factor receptor as a novel therapeutic target in anaplastic thyroid carcinomas. Ann N Y Acad Sci 2004; 1030:69 - 77; http://dx.doi.org/10.1196/annals.1329.009; PMID: 15659782
- Elliott DD, Sherman SI, Busaidy NL, Williams MD, Santarpia L, Clayman GL, et al. Growth factor receptors expression in anaplastic thyroid carcinoma: potential markers for therapeutic stratification. Hum Pathol 2008; 39:15 - 20; http://dx.doi.org/10.1016/j.humpath.2007.05.012; PMID: 17949783
- Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg 2001; 25:617 - 22; http://dx.doi.org/10.1007/s002680020166; PMID: 11369989
- Aratake Y, Nomura H, Kotani T, Marutsuka K, Kobayashi K, Kuma K, et al. Coexistant anaplastic and differentiated thyroid carcinoma an immunohistochemical study. Am J Clin Pathol 2006; 125:399 - 406
- Wang HM, Huang YW, Huang JS, Wang CH, Kok VC, Hung CM, et al. Anaplastic carcinoma of the thyroid arising more often from follicular carcinoma than papillary carcinoma. Ann Surg Oncol 2007; 14:3011 - 8; http://dx.doi.org/10.1245/s10434-007-9503-8; PMID: 17638058
- Wiseman SM, Griffith OL, Deen S, Rajput A, Masoudi H, Gilks B, et al. Identification of molecular markers altered during transformation of differentiated into anaplastic thyroid carcinoma. Arch Surg 2007; 142:717 - 27, discussion 727-9; http://dx.doi.org/10.1001/archsurg.142.8.717; PMID: 17709725
- Wiseman SM, Masoudi H, Niblock P, Turbin D, Rajput A, Hay J, et al. Derangement of the E-cadherin/catenin complex is involved in transformation of differentiated to anaplastic thyroid carcinoma. Am J Surg 2006; 191:581 - 7; http://dx.doi.org/10.1016/j.amjsurg.2006.02.005; PMID: 16647341
- Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer 2005; 103:2261 - 8; http://dx.doi.org/10.1002/cncr.21073; PMID: 15880523
- Montero-Conde C, Martín-Campos JM, Lerma E, Gimenez G, Martínez-Guitarte JL, Combalía N, et al. Molecular profiling related to poor prognosis in thyroid carcinoma. Combining gene expression data and biological information. Oncogene 2008; 27:1554 - 61; http://dx.doi.org/10.1038/sj.onc.1210792; PMID: 17873908
- Elliott DD, Sherman SI, Busaidy NL, Williams MD, Santarpia L, Clayman GL, et al. Growth factor receptors expression in anaplastic thyroid carcinoma: potential markers for therapeutic stratification. Hum Pathol 2008; 39:15 - 20; http://dx.doi.org/10.1016/j.humpath.2007.05.012; PMID: 17949783
- Mitsiades CS, Kotoula V, Poulaki V, Sozopoulos E, Negri J, Charalambous E, et al. Epidermal growth factor receptor as a therapeutic target in human thyroid carcinoma: mutational and functional analysis. J Clin Endocrinol Metab 2006; 91:3662 - 6; http://dx.doi.org/10.1210/jc.2006-0055; PMID: 16822827
- Lee DH, Lee GK, Kong SY, Kook MC, Yang SK, Park SY, et al. Epidermal growth factor receptor status in anaplastic thyroid carcinoma. J Clin Pathol 2007; 60:881 - 4; http://dx.doi.org/10.1136/jcp.2006.041251; PMID: 17079354
- Ulisse S, Delcros JG, Baldini E, Toller M, Curcio F, Giacomelli L, et al. Expression of Aurora kinases in human thyroid carcinoma cell lines and tissues. Int J Cancer 2006; 119:275 - 82; http://dx.doi.org/10.1002/ijc.21842; PMID: 16477625
- Sorrentino R, Libertini S, Pallante PL, Troncone G, Palombini L, Bavetsias V, et al. Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J Clin Endocrinol Metab 2005; 90:928 - 35; http://dx.doi.org/10.1210/jc.2004-1518; PMID: 15562011